Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, market expansion faces significant hurdles due to the high costs associated with long-term treatment plans, which complicate reimbursement processes on a global scale. Constrained healthcare budgets often necessitate strict utilization management strategies that can delay or deny patient access to this class of drugs. Data from the International Society for Pharmacoeconomics and Outcomes Research indicates that in 2024, patients undergoing continuous treatment with thrombopoietin receptor agonists incurred mean total healthcare costs of approximately $162,253. This substantial financial burden presents a formidable challenge to ensuring equitable drug availability across diverse economic regions.
Market Drivers
The increasing prevalence of chronic immune thrombocytopenia (ITP) acts as a primary catalyst for the Global Eltrombopag Drugs Market, directly enlarging the patient population that requires long-term platelet management. As the incidence of this autoimmune condition rises, the reliance on thrombopoietin receptor agonists to maintain safe platelet levels intensifies, driving consistent pharmaceutical consumption. According to a November 2024 report in Blood titled 'Real-World Retrospective Analysis of Immune Thrombocytopenia Patient Demographics and Treatment', the incidence of immune thrombocytopenia was recorded at 6.1 per 100,000 people. This growing disease burden generates substantial commercial value for established therapies, evidenced by Novartis reporting 2024 annual net sales of approximately $2.2 billion for Promacta/Revolade, highlighting the significant financial scale achieved through treating this expanding patient base.The broadening of regulatory approvals for pediatric and first-line use further stimulates market growth by transitioning eltrombopag from a salvage therapy to a standard early intervention. Clinical evidence demonstrating superior efficacy compared to traditional immunosuppressive regimens continues to support these regulatory advancements, encouraging healthcare providers to adopt the drug for newly diagnosed severe aplastic anemia. As noted in the Annals of Hematology in August 2024, within the article 'Comparison of efficacy of eltrombopag combined with immunosuppression..', the complete response rate at six months for patients treated with eltrombopag in a first-line setting was 31.3%, compared to 19.4% for those on standard therapy alone. Such data validates the drug's utility in early-stage treatment protocols, thereby expanding its application scope across diverse age groups and disease severities.
Market Challenges
The high expense associated with prolonged Eltrombopag treatment regimens poses a severe restriction on the growth of the global market. As healthcare systems worldwide operate under increasingly tighter budgets, the elevated pricing of this thrombopoietin receptor agonist creates significant friction within reimbursement processes. Payers and insurance providers frequently implement strict utilization management strategies, such as rigorous prior authorization requirements or step-therapy protocols, which delay or outright deny patient access to the drug. This financial barrier effectively shrinks the addressable market by excluding patients who lack comprehensive insurance coverage or reside in regions with capped healthcare expenditure.The magnitude of this financial burden is highlighted by recent data regarding specific treatment indications. According to the American Society of Hematology, in 2024, the cost associated with a six-month course of Eltrombopag for severe aplastic anemia was estimated to be approximately $157,000. Such prohibitive costs force healthcare providers to reserve this effective therapy for only the most severe or refractory cases, thereby limiting widespread adoption. Consequently, these pricing pressures hamper market penetration and suppress the commercial revenue potential of the drug in both developed and emerging economies.
Market Trends
The commercialization of cost-effective generic Eltrombopag formulations is reshaping the market by dismantling pricing barriers that have historically restricted patient access. Following the expiration of key patents for the branded therapy, regulatory bodies are accelerating approvals of bioequivalent alternatives, which introduces significant price competition and alters reimbursement strategies globally. This influx forces incumbent manufacturers to adjust pricing models while simultaneously expanding the addressable patient pool in cost-sensitive regions where high-cost brands were previously unaffordable. For instance, in October 2024, the European Medicines Agency recommended granting marketing authorization for Eltrombopag Viatris, a generic formulation indicated for primary immune thrombocytopenia and severe aplastic anemia, signaling the formal entry of lower-cost competitors into the European sector.Intensifying competition from newer generation TPO-receptor agonists is concurrently challenging Eltrombopag’s dominance through improved convenience profiles and efficacy. Second-generation agents such as avatrombopag are gaining clinical preference because they eliminate the strict dietary restrictions associated with eltrombopag, specifically the requirement to avoid calcium-rich foods during administration. This competitive pressure is redirecting market share away from established therapies as healthcare providers favor options that simplify patient adherence and reduce lifestyle burdens. According to Sobi’s October 2024 interim report, revenue for the competing drug Doptelet increased by 65 percent at constant exchange rates to SEK 1.03 billion compared to the previous year, demonstrating the swift commercial uptake of these newer therapeutic alternatives.
Key Players Profiled in the Eltrombopag Drugs Market
- Novartis AG
- GlaxoSmithKline PLC
- Teva Pharmaceutical Industries Ltd.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
- Annora Pharma Pvt Ltd.
- Ningbo Menovo Pharmaceutical Co., Ltd.
- Guangdong Longfu Pharmaceutical Co., Ltd.
- Grand Pharmaceutical Group Limited
- Qilu Pharmaceutical Co., Ltd.
- Sichuan Kelun Pharmaceutical Co., Ltd.
Report Scope
In this report, the Global Eltrombopag Drugs Market has been segmented into the following categories:Eltrombopag Drugs Market, by Type:
- Tablets
- Oral Suspension
Eltrombopag Drugs Market, by Application:
- Chronic Immune Thrombocytopenia
- Hepatitis C
- Severe Aplastic Anemia
- Others
Eltrombopag Drugs Market, by End User:
- Hospitals and Clinics
- Pharmacies
- Others
Eltrombopag Drugs Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Eltrombopag Drugs Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Eltrombopag Drugs market report include:- Novartis AG
- GlaxoSmithKline PLC
- Teva Pharmaceutical Industries Ltd.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
- Annora Pharma Pvt Ltd.
- Ningbo Menovo Pharmaceutical Co., Ltd.
- Guangdong Longfu Pharmaceutical Co., Ltd.
- Grand Pharmaceutical Group Limited
- Qilu Pharmaceutical Co., Ltd.
- Sichuan Kelun Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
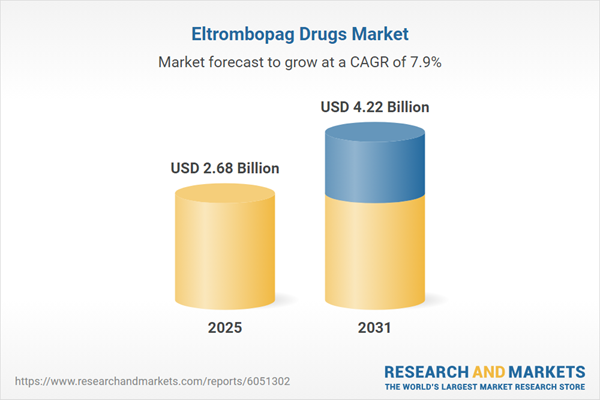
| Estimated Market Value ( USD | $ 2.68 Billion |
| Forecasted Market Value ( USD | $ 4.22 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |