Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite strong demand, the market encounters a significant obstacle due to the high cost of therapy associated with echinocandin-based drugs relative to conventional azoles. The intricate fermentation and semi-synthetic manufacturing processes needed to produce the Echinocandin B nucleus lead to elevated production costs, which are inevitably reflected in the final pricing. This financial barrier hinders the widespread adoption of these therapeutics in price-sensitive developing regions with limited healthcare budgets, thereby constraining the overall potential for market expansion.
Market Drivers
The increasing prevalence of multidrug-resistant fungal pathogens is the primary force accelerating the Global Echinocandin B Market. As immunocompromised patient populations grow due to rising rates of organ transplants and oncological therapies, susceptibility to severe mycoses has intensified, necessitating robust supply chains for echinocandin intermediates.This urgency is highlighted by the rapid spread of pathogens such as Candida auris, which often displays resistance to traditional azole therapies, thus prioritizing echinocandin-class drugs as a first-line defense. According to the December 2024 'Tracking C. auris' surveillance report by the Centers for Disease Control and Prevention, clinical cases of this multidrug-resistant fungus in the U.S. reached 4,514 in 2023, indicating a continued upward trend that directly increases manufacturing requirements for effective lipopeptide-based antifungals.
Simultaneously, the expansion of pharmaceutical R&D for new antifungal formulations is reshaping market dynamics by driving demand for high-purity Echinocandin B nuclei. Pharmaceutical developers are actively commercializing next-generation echinocandins with enhanced stability and dosing schedules, a process heavily reliant on advanced semi-synthetic manufacturing. In May 2024, Cidara Therapeutics reported in its 'First Quarter 2024 Financial Results' that it received a milestone payment of approximately $11.1 million from Mundipharma following the European Union approval of the novel echinocandin rezafungin, confirming the commercial viability of these advanced therapies. Further reflecting confidence in this therapeutic area, F2G secured a $100 million financing round in 2024 led by the AMR Action Fund to advance late-stage antifungal development, underscoring the significant global investment entering the sector.
Market Challenges
The high cost of therapy associated with echinocandin-based drugs presents a significant hurdle to market expansion, particularly in cost-sensitive developing regions. Producing the Echinocandin B nucleus involves complex fermentation and semi-synthetic processes that inherently increase manufacturing expenses. These elevated costs are transferred to the final pricing, establishing a substantial financial barrier for healthcare systems with restricted budgets. Consequently, medical providers in these areas often resort to conventional, lower-cost azoles despite the clinical benefits of echinocandins, thereby limiting the addressable market for these advanced therapeutics.This price sensitivity is further exacerbated by the broader economic strain that fungal diseases place on healthcare infrastructure, forcing administrators to strictly manage pharmacy expenditures. According to the Infectious Diseases Society of America, the total annual economic burden of fungal diseases in the United States was estimated to be approximately $19.4 billion in 2024. Such immense financial pressure compels healthcare institutions to prioritize cost containment, further suppressing the adoption rates of premium echinocandin treatments in favor of more affordable alternatives, which directly hampers volume growth in the Global Echinocandin B Market.
Market Trends
The rise of next-generation long-acting derivatives is fundamentally altering the commercial landscape for echinocandin intermediates, shifting the market from daily formulations like anidulafungin toward weekly dosing regimens. This transition requires the Echinocandin B nucleus to serve as a scaffold for more advanced chemical modifications, thereby increasing the technical complexity and value of the supply chain. Pharmaceutical companies are increasingly divesting these capital-intensive development programs to partners with specialized commercial infrastructure to maximize market penetration. For instance, Cidara Therapeutics projected in its 'Second Quarter 2024 Financial Results' in August 2024 that it would achieve approximately $128 million in cost savings over the patent life of rezafungin following the strategic divestiture of the asset to Mundipharma for global commercialization, illustrating the industry-wide push to realign resources toward these high-value, next-generation therapies.At the same time, the market is experiencing a rapid expansion of generic anidulafungin API production, driven by the expiration of key patents and the subsequent entry of large-scale sterile injectable manufacturers. This trend is democratizing access to echinocandin therapies, forcing incumbent API producers to optimize fermentation yields to compete on price while meeting the stringent purity standards required for generic regulatory approvals. The intense volume growth in this segment benefits manufacturers with vertically integrated capabilities who can sustain margins despite pricing pressures. In May 2024, Gland Pharma reported a 56% year-over-year increase in consolidated revenue in its 'Financial Results for the Fourth Quarter and Full Year ended March 31, 2024', a surge attributed to robust volume growth in its core sterile injectable markets, validating the accelerating global demand for cost-effective generic antifungal formulations.
Key Players Profiled in the Echinocandin B Market
- Glenmark Pharmaceuticals Ltd.
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Mylan N.V.
- Fresenius SE & Co. KGaA
- Merck & Co., Inc.
- Astellas Pharma Inc.
- Pfizer Inc.
- Cidara Therapeutics, Inc.
- Hikma Pharmaceuticals PLC
Report Scope
In this report, the Global Echinocandin B Market has been segmented into the following categories:Echinocandin B Market, by Type:
- Powder
- Liquid
Echinocandin B Market, by Application:
- Antifungal Drugs
- Drug Development
- Others
Echinocandin B Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Echinocandin B Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Echinocandin B market report include:- Glenmark Pharmaceuticals Ltd.
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Mylan N.V.
- Fresenius SE & Co. KGaA
- Merck & Co., Inc.
- Astellas Pharma Inc.
- Pfizer Inc.
- Cidara Therapeutics, Inc.
- Hikma Pharmaceuticals PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
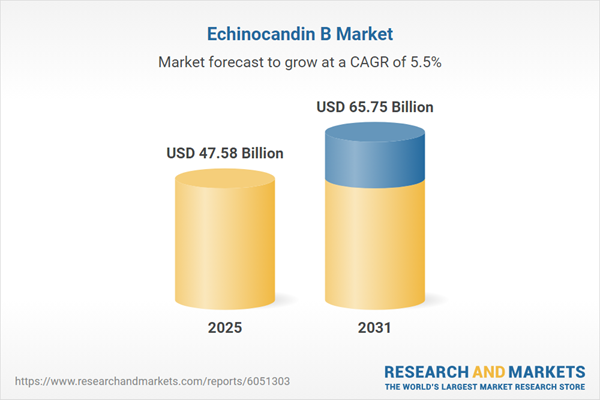
| Estimated Market Value ( USD | $ 47.58 Billion |
| Forecasted Market Value ( USD | $ 65.75 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |