Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market's upward trajectory encounters a significant obstacle regarding the risk of major bleeding events linked to these potent medications. Although reversal agents have been developed, their high acquisition costs and limited availability in emerging economies often discourage widespread implementation. This economic barrier presents a substantial challenge to the universal adoption of these sophisticated anticoagulant therapies.
Market Drivers
The escalating prevalence of venous thromboembolism and atrial fibrillation, particularly within an aging global demographic, serves as the primary catalyst for market growth. As life expectancy increases, the incidence of these thromboembolic conditions rises markedly, creating a need for effective long-term anticoagulation strategies to lower the risk of systemic embolism and stroke. This demographic shift generates a consistent and growing patient pool requiring chronic therapeutic intervention. Highlighting the increasing burden of these conditions, the Centers for Disease Control and Prevention projected in a May 2024 report that 12.1 million individuals in the United States will suffer from atrial fibrillation by 2030. This forecast underscores the urgent necessity for accessible and effective treatments to address the risks associated with this expanding vulnerable population.Concurrently, the market is driven by the superior clinical profile and convenience these therapies offer compared to traditional Vitamin K antagonists. Healthcare providers and patients are increasingly prioritizing these newer agents because they provide predictable pharmacokinetics and eliminate the burdensome requirement for routine International Normalized Ratio monitoring. This broad shift in clinical preference is quantitatively demonstrated by the commercial success of leading drugs in this class. For instance, Bristol Myers Squibb reported in February 2024 that its flagship oral anticoagulant generated worldwide revenues of $12.2 billion, signaling robust demand for these advanced therapeutic options. Given that the World Stroke Organization estimated in 2024 that over 12 million strokes occur globally each year, the safety assurance provided by these medications remains critical for effective preventative anticoagulation.
Market Challenges
The growth of the Global DOACs Market is significantly hindered by the inherent risk of major bleeding events associated with these powerful therapies. The potential for uncontrolled hemorrhaging, specifically intracranial and gastrointestinal bleeding, creates considerable hesitation among clinicians when prescribing for high-risk patient groups. This caution is compounded by the economic burden of safety management; although reversal agents are available, their prohibitive acquisition costs often render them inaccessible within many healthcare systems. Consequently, providers in cost-constrained environments frequently forgo these advanced agents to avoid the financial risks associated with managing potential complications.The magnitude of this safety concern is underscored by recent data highlighting the strain on healthcare services. According to the Centers for Disease Control and Prevention in 2024, anticoagulants were the leading cause of emergency department visits for adverse drug events, accounting for approximately 21% of these critical encounters. This high frequency of medication-related emergencies necessitates resource-intensive hospitalizations, directly offsetting the perceived clinical advantages of DOACs. The convergence of clinical safety risks and the high cost of emergency reversal therapies continues to restrict market penetration, particularly in developing economies.
Market Trends
The introduction of generic DOAC formulations following patent expirations is fundamentally reshaping the competitive landscape, particularly in European markets where exclusivity periods for major brands have recently concluded. This structural shift is forcing a transition from high-margin branded therapies to cost-effective bioequivalent alternatives, significantly impacting the revenue streams of established pharmaceutical leaders. As regulatory bodies increasingly approve these generic options, the market is experiencing widespread price erosion, which paradoxically expands patient access in cost-sensitive regions while challenging the commercial dominance of originator drugs. The financial implication of this trend is already visible; Bristol Myers Squibb reported in February 2024 that international revenues for its leading anticoagulant Eliquis were $975 million, representing a 3% decrease adjusted for foreign exchange, primarily driven by lower average net selling prices and generic erosion in several European countries.Simultaneously, the clinical development of next-generation Factor XI inhibitors represents a pivotal advancement aimed at decoupling hemostasis from thrombosis management. Unlike current DOACs that carry a risk of major bleeding, these novel agents are designed to prevent pathologic thrombus formation while sparing the coagulation mechanisms necessary for injury repair. This safety-centric innovation addresses the critical unmet need for anticoagulants that can be safely administered to high-risk populations, such as those requiring concomitant antiplatelet therapy. The clinical promise of this class is substantiated by recent trial results; in November 2024, Anthos Therapeutics reported that the novel Factor XI inhibitor abelacimab demonstrated a substantial 67% reduction in bleeding events for patients on antiplatelet therapy compared to the standard-of-care rivaroxaban, highlighting its potential to redefine safety standards.
Key Players Profiled in the Direct Oral Anticoagulants (DOACs) Market
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Pfizer Inc.
- Sanofi
- Zhejiang Huahai Pharmaceutical Co. Ltd.
- Janssen Global Services, LLC
- Aspen Pharmacare Holdings Limited
- Astellas Pharma Inc.
- Lupin Pharmaceutical, Inc.
Report Scope
In this report, the Global Direct Oral Anticoagulants (DOACs) Market has been segmented into the following categories:Direct Oral Anticoagulants (DOACs) Market, by Drug Type:
- Factor Xa Inhibitors
- Direct Thrombin Inhibitors
Direct Oral Anticoagulants (DOACs) Market, by Indication:
- Atrial Fibrillation (AF)
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism (PE)
- Stroke Prevention
- Others
Direct Oral Anticoagulants (DOACs) Market, by End User:
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Home Care Settings
- Others
Direct Oral Anticoagulants (DOACs) Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Direct Oral Anticoagulants (DOACs) Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Direct Oral Anticoagulants (DOACs) market report include:- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Pfizer Inc.
- Sanofi
- Zhejiang Huahai Pharmaceutical Co. Ltd.
- Janssen Global Services, LLC
- Aspen Pharmacare Holdings Limited
- Astellas Pharma Inc.
- Lupin Pharmaceutical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
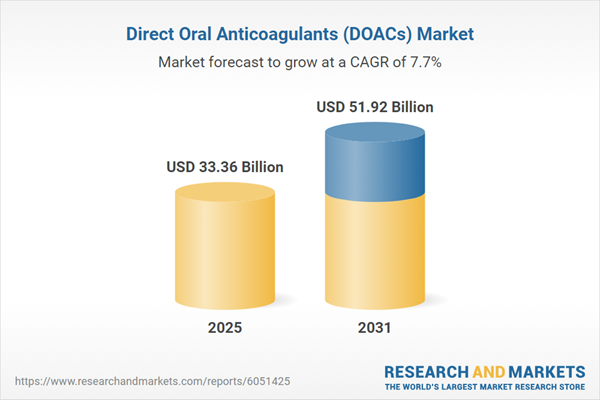
| Estimated Market Value ( USD | $ 33.36 Billion |
| Forecasted Market Value ( USD | $ 51.92 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |