Oncolytic Virus Cancer Therapy Market Overview
Oncolytic viruses refer to a form of immunotherapy where viruses are used to infect and destroy cancer cells. These viruses are genetically modified to selectively infect and replicate within cancer cells, which minimizes the damage to healthy cells. The market for oncolytic virus cancer therapy is driven by the rising incidence of cancer and growing awareness among patients and healthcare providers about this treatment option. The increasing investment in the development of oncolytic virus therapies from both public and private sectors is also supporting the market growth. Further, the growing emphasis on the development of next-generation oncolytic viruses and rising preference for combination therapies is likely to impact the market dynamics.Oncolytic Virus Cancer Therapy Market Growth Drivers
Rising Incidence of Cancer to Drive the Market Growth
According to the National Cancer Institute, around 2,002,140 new cases of cancer are estimated to be diagnosed in the United States in 2024. Moreover, about 611,720 individuals are projected to die from the disease. Further, it is estimated that 14,910 children and adolescents between the ages of 0 to 19 will be diagnosed with cancer in the country in 2024, with nearly 1,590 of them predicted to die from the disease. Thus, the rise in cancer cases is generating a strong demand for innovative treatments like oncolytic virus therapies, which is anticipated to contribute to the market growth.Oncolytic Virus Cancer Therapy Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Supportive Regulatory Framework to Affect the Market Landscape Significantly
In November 2023, a modified oncolytic vaccinia virus Olvi-Vec (olvimulogene nanivacirepvec) received fast-track designation from the US Food and Drug Administration (FDA) for the treatment of patients with platinum-resistant/refractory ovarian cancer. The oncolytic virus-based cancer therapy is developed by clinical-stage biopharmaceutical company Genelux Corporation and is designed to selectively target malignant cells. Such regulatory support is expected to expedite the development and approval process, thereby aiding market growth.Growth of Combination Therapies Poised to Augment Oncolytic Virus Cancer Therapy Market Demand
One of the significant market trends is the growing use of oncolytic viruses in combination with other cancer treatments like chemotherapy, radiation, CAR-T cell therapies, and immune checkpoint inhibitors. This trend is driven by the growth in clinical trials that aim to evaluate and optimize these combinations to enhance patient outcomes in the coming years.Increasing Investments to Elevate the Oncolytic Virus Cancer Therapy Market Value
In February 2023, the clinical-stage biotechnology company TILT Biotherapeutics announced the final close of its financing round led by Finland’s Lifeline Ventures, where it secured Euro 22 million (around USD 23.8 million). The company intends to use the funds to advance the Phase II trial of its oncolytic immunotherapies that are used synergistically with checkpoint inhibitors. Such substantial investment initiatives are likely to elevate the market value in the forecast period and support the development of novel oncolytic virus treatments.Advancement in Genetic Engineering to Boost Oncolytic Virus Cancer Therapy Market Size
The market benefits from the rising advancements in genetic engineering that are leading to the development of highly specialized viruses that can target specific cancer cells. The availability of advanced techniques such as CRISPR and other gene-editing tools allows for precise modifications that not only increase the selectivity but also the safety of oncolytic viruses, thereby driving the development of more effective and safer therapies.Oncolytic Virus Cancer Therapy Market Segmentation
"Oncolytic Virus Cancer Therapy Market Report and Forecast 2025-2034" offers a detailed analysis of the market based on the following segments:Market Breakup by Virus Type
- Genetically Engineered Oncolytic Viruses
- Adenovirus
- Vaccinia Virus
- Others
- Oncolytic Wild-type Viruses
- Reovirus
- Newcastle Disease Virus
- Vesicular Stomatitis Virus
- Others
Market Breakup by Application
- Solid Tumor
- Breast Cancer
- Prostate Cancer
- Lung Cancer
- Glioblastoma
- Melanoma
- Hematological Malignancies
- Lymphoma
- Leukemia
- Others
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Cancer Research Institutes
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- India
- Japan
Oncolytic Virus Cancer Therapy Market Share
The Virus Type Segment Holds a Substantial Market Share
Based on the virus type, the market is segmented into genetically engineered oncolytic viruses including adenovirus, and vaccinia virus, among others, and oncolytic wild-type viruses which comprise reovirus, Newcastle disease virus, vesicular stomatitis virus, and others. The genetically engineered oncolytic viruses segment covers a significant market share as these viruses can be modified to improve their ability to target cancer cells and minimize damage to healthy cells. This customization and increased selectivity of the oncolytic viruses that are genetically engineered allows for better efficacy and safety, thereby contributing to market growth.Oncolytic Virus Cancer Therapy Market Analysis by Region
The market segmentation by region includes the United States, EU-4 (Germany, France, Italy, Spain), and the United Kingdom, Japan, and India. The United States dominates the market for oncolytic virus cancer therapy, owing to a significant focus on cancer research and substantial public and private investment in oncolytic virus therapies. The presence of major market players and research institutions actively involved in the development and clinical trials of oncolytic virus therapies further supports the market growth in the region. Moreover, the advanced healthcare infrastructure and high prevalence rate of cancer in the country are poised to fuel the market expansion.Leading Players in the Oncolytic Virus Cancer Therapy Market
The key features of the market report comprise patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, and strategic initiatives by the leading key players. The major companies in the market are as follows:Theriva Biologics
Theriva Biologics (formerly Synthetic Biologics, Inc.), headquartered in Maryland, United States, is a clinical-stage immuno-oncology company specializing in the development of oncolytic viruses that can overcome the protective barrier present around solid tumors and specifically target tumor cells. One of Theriva’s lead candidates includes an oncolytic adenovirus VCN-1 which is designed to aggressively and selectively replication within tumor cells.TILT Biotherapeutics Oy
Oncolytic immunotherapy startup company TILT Biotherapeutics Oy is one of the key players in the market known for developing intravenous next-generation oncolytic immunotherapies. The company’s patented TILT® technology, which is based on oncolytic viral therapies, is designed to modify the tumor microenvironment and destroy its ability to affect immune responses.Boehringer Ingelheim International GmbH
Boehringer Ingelheim International GmbH, headquartered in Ingelheim am Rhein, Germany, is a global pharmaceutical company. It is involved in the development of innovative cancer treatments including oncolytic virus therapies. The company is also focused on exploring oncolytic viruses in combination with other immuno-oncology agents.Recipharm AB
Sweden-based Recipharm AB is a leading pharmaceutical contract development and manufacturing organization known for offering services in the production and development of biologics, including oncolytic viruses. The company invests heavily in facilities and technologies, boasting production facilities in the United Kingdom, France, Germany, Italy, India, and other key regions along with development sites in Israel, the United States, and Sweden.Other key players in the market include ONCOVITA, ViraTherapeutics GmbH, Amgen Inc., and Biotherapy International.
Key Questions Answered in the Oncolytic Virus Cancer Therapy Market Report
- What was the oncolytic virus cancer therapy market value in 2024?
- What is the oncolytic virus cancer therapy market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on virus type?
- What is the market breakup based on application?
- Who are the major end users in the market?
- What are the major factors aiding the oncolytic virus cancer therapy market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- How do the prevalence and incidence of cancer affect the market landscape?
- What are the major oncolytic virus cancer therapy market trends?
- How does the favorable regulatory environment impact the market size?
- Which virus type will dominate the market share?
- Which application is expected to have a high market value in the coming years?
- Which end user is projected to contribute to the highest market growth?
- Who are the key players involved in the oncolytic virus cancer therapy market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Theriva Biologics
- TILT Biotherapeutics Oy
- Boehringer Ingelheim International GmbH
- Recipharm AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
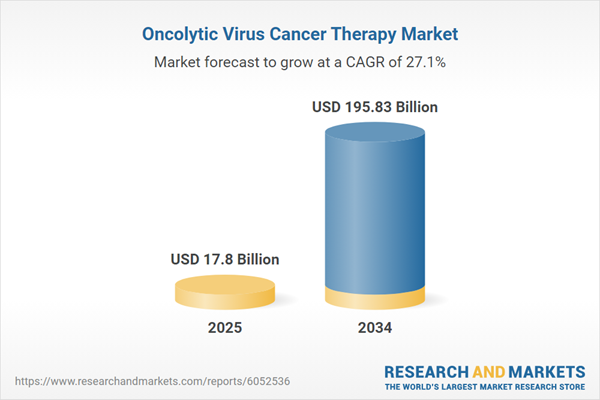
| Estimated Market Value ( USD | $ 17.8 Billion |
| Forecasted Market Value ( USD | $ 195.83 Billion |
| Compound Annual Growth Rate | 27.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |