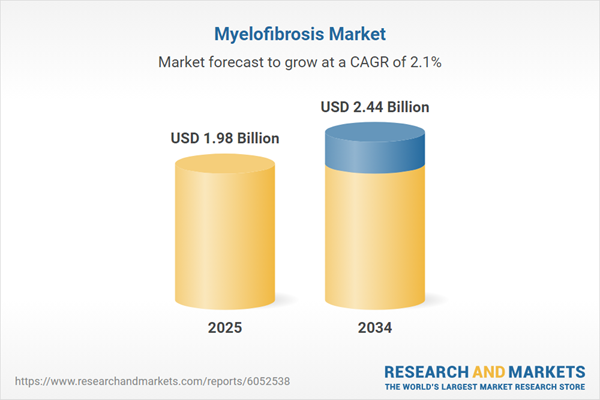
Myelofibrosis Market Overview
Myelofibrosis is a rare blood cancer that disrupts the normal production of blood cells in the body. It causes severe bone marrow scarring and reduces the number of blood clotting cells called platelets, leading to weakness and fatigue. There are two types including primary myelofibrosis and secondary myelofibrosis.Myelofibrosis Market Growth Drivers
Rising Prevalence of Myelofibrosis Boosts Growth
The rising incidence of the condition is playing an important role in the increasing demand for effective and innovative treatments for the condition. The Leukemia and Lymphoma Society Report 2022-2023 revealed that nearly 157,561 people are living with or in remission from myeloma in the United States. It was estimated that about 35,730 people would be diagnosed with Myeloma in 2023 and approximately 12,590 people are expected to die from myeloma.Increasing Drug Approvals to Meet Myelofibrosis Market Demand
The market growth is expected to be bolstered by the rising regulatory approvals by the authorities. These approvals play a pivotal role in the availability of the latest treatment drugs and therapies in the market, contributing to enhanced disease management and patient outcomes. For instance, in June 2024, GlaxoSmithKline announced that the Ministry of Health, Labour, and Welfare in Japan has approved momelotinib (Omjjara) for the treatment of patients suffering from myelofibrosis. The regulatory approval was supported by data from the phase 3 MOMENTUM (NCT04173494) and SIMPLIFY-1 (NCT01969838) trials. The approval of momelotinib (Omjjara) is expected to play a crucial role in the treatment of myelofibrosis as it offers a new treatment option, especially for patients with anemia. This could potentially improve patient outcomes and quality of life, triggering competition among the market players.Moreover, in January 2024, the regulation of momelotinib (Omjjara) was approved by the European Commission. It is the first agent for the treatment of adult patients with disease-related splenomegaly or symptoms and moderate to severe anemia including patients with primary myelofibrosis, post-polycythemia vera (PV) myelofibrosis, or post-essential thrombocythemia (ET) myelofibrosis and who are JAK inhibitor naive or have treatment history with ruxolitinib (Jakafi). The approval of the once-a-day, oral JAK1/JAK2, and ACVR1 inhibitor was based on data from the phase 3 MOMENTUM trial (NCT04173494) and data from a subpopulation of patients with moderate to severe anemia from the phase 3 SIMPLIFY-1 trial (NCT01969838).
Myelofibrosis Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increased Drug Approvals
The market is driven by the rising number of drug approvals for myelofibrosis, such as JAK inhibitors and novel therapies like momelotinib. The approvals are poised to drive market growth as they are enabling new treatment options to be available in the market for use by healthcare providers.Advancement in Precision Medicine
Advancements in precision medicine are enhancing the myelofibrosis market by enabling targeted therapies that improve treatment efficacy. Personalised treatments based on genetic profiling tend to provide enhanced patient outcomes, contributing to the adoption of advanced therapies.Growing Awareness and Early Diagnosis
The market is experiencing increased awareness of myelofibrosis and advancements in diagnostic techniques, leading to earlier diagnosis, and improved patient outcomes. Increasing early diagnosis contributes to the rising demand for innovative and effective therapies, stimulating market growth.Escalating Research and Clinical Trials
The market is experiencing a surge in research and clinical trials focused on myelofibrosis, bolstering market growth. These activities are leading to the development of improved therapies and increased understanding of the disease, which in turn may help and accelerate drug discoveries, fuelling market expansion.Myelofibrosis Market Segmentation
Market Breakup by Type
- Primary Myelofibrosis
- Secondary Myelofibrosis
Market Breakup by Diagnosis
- Bone Marrow Biopsy
- Genetic Tests
- Blood Tests
- Others
Market Breakup by Treatment Type
- Target Therapy
- Chemotherapy
- Stem Cell Transplant
- Others
Market Breakup by End User
- Hospitals and Clinics
- Specialty Clinics
- Cancer Centers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Myelofibrosis Market Share
Market Share by Type to Undergo Growth
The market segmentation by type includes primary myelofibrosis and secondary myelofibrosis. The market share is dominated by primary myelofibrosis due to its higher prevalence and increased focus of researchers on developing targeted therapies for the condition. Primary myelofibrosis is a chronic condition with significant unmet needs, driving demand for effective treatments and diagnostics. The market dominance can also be attributed to the increasing availability of novel therapies such as JAK inhibitors, which are primarily indicated for the condition. Secondary myelofibrosis is quite uncommon and often occurs as a progression from myeloproliferative disorders.Target Therapy to Lead the Share Based on Treatment Type
On the basis of treatment type, the segmentation includes target therapy, chemotherapy, stem cell transplant, and others. The market share is expected to be led by the targeted therapy due to its ability to specifically address the genetic mutations that support the disease. JAK inhibitors are a key component of targeted therapy and are effective in managing symptoms and improving patient outcomes. Targeted therapies offer a better safety profile and fewer side effects compared to traditional chemotherapy. These treatments are more suitable for long-term treatments which are prominent in managing chronic conditions like myelofibrosis. The continuous development and approval of new targeted drugs is boosting the market dominance.Myelofibrosis Market Analysis by Region
The United States dominates the regional market share due to the presence of a well-established healthcare infrastructure, high prevalence of the condition, and continuous research and development activities by the researchers. With the presence of an advanced healthcare infrastructure, the region experiences increased adoption of advanced technologies and the development of effective therapies. The presence of leading market players and access to cutting-edge diagnostic and treatment options contribute to the dominance of the United States. The market dominance is further fuelled by the increased awareness of myelofibrosis and healthcare policies available in the region to support the treatment of the condition. Heavy investments by the market players in the development of effective treatment therapies for myelofibrosis are propelling overall market growth in the region.Leading players in the Myelofibrosis Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis and strategic initiatives by the leading players. The major companies in the market are as follows:Cleveland Clinic
Cleveland Clinic is a renowned nonprofit academic medical center known for its cutting-edge research and treatment of various diseases, including myelofibrosis. With a strong focus on innovative healthcare, it offers advanced diagnostic and therapeutic options for hematologic cancers. Its multidisciplinary approach integrates clinical trials, targeted therapies, and bone marrow transplants, making it a leader in the treatment of rare blood disorders like myelofibrosis.GSK plc
GSK plc (GlaxoSmithKline) is a global healthcare company actively involved in the myelofibrosis market through its development of targeted therapies and immuno-oncology drugs. The company’s oncology portfolio includes JAK inhibitors and other molecular treatments aimed at improving survival rates in myelofibrosis patients. GSK's commitment to R&D is evident in its focus on innovative treatments for rare hematologic cancers.Herbert Irving Comprehensive Cancer Center
Herbert Irving Comprehensive Cancer Center, affiliated with Columbia University, is a leading institution in cancer research and treatment. It offers specialised care for myelofibrosis patients, focusing on clinical trials, molecular therapies, and stem cell transplantation. The center is dedicated to enhance the understanding and treatment of myeloproliferative neoplasms, positioning itself as a key player in innovative myelofibrosis therapies.SHEBA Medical Center
SHEBA Medical Center, Israel's largest hospital, is a premier healthcare institution with a strong oncology department specializing in hematologic cancers like myelofibrosis. Known for its research in bone marrow transplants and cellular therapies, SHEBA provides cutting-edge treatment options and personalized care. The center is involved in clinical trials and experimental treatments, contributing significantly to advancements in the management of myelofibrosis.SUN Pharmaceutical Ltd
SUN Pharmaceutical Ltd is a global pharmaceutical company with a growing presence in the myelofibrosis market. It focuses on developing and marketing generic and branded oncology drugs, including those targeting hematologic disorders. The company's research in targeted therapies and its expanding portfolio of cancer medications make it a notable player in providing treatment options for myelofibrosis and other blood-related cancers.Key Questions Answered in the Myelofibrosis Market
- What was the myelofibrosis market value in 2024?
- What is the myelofibrosis market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on type?
- What is market segmentation based on treatment type?
- How is the market segmented based on the diagnosis?
- Who are the end-users in the market?
- What are the major factors aiding the myelofibrosis market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major myelofibrosis market trends?
- Which technology is expected to dominate the market?
- Which type will lead the market segment?
- Which treatment type will dominate the market share?
- Which diagnosis is poised to lead the market share?
- Which end-user will lead the market?
- Who are the key players involved in the myelofibrosis market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Cleveland Clinic
- GSK plc
- Herbert Irving Comprehensive Cancer Center
- SHEBA Medical Center
- SUN Pharmaceutical Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.98 Billion |
| Forecasted Market Value ( USD | $ 2.44 Billion |
| Compound Annual Growth Rate | 2.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |