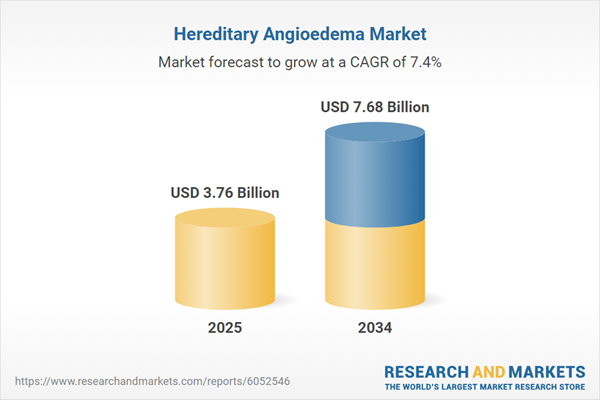
Hereditary Angioedema Market Overview
Hereditary angioedema is a disorder in which the patient experiences recurrent episodes of severe swelling in various body parts such as limbs, face, intestinal tract, and airway. Episodes that affect the intestinal tract may cause severe abdominal pain, nausea, and vomiting. Further, a swollen airway can restrict breathing and lead to life-threatening obstruction of the airway. It is also common for the patient with this condition to develop a non-itchy rash called erythema marginatum during an attack.Hereditary Angioedema Market Growth Drivers
FDA Approvals Contributing to Increased Treatment Options and Cost Efficiency
The market growth can be attributed to the rising research and development activities by the researchers backed by key market players and governments. The rise in these clinical studies is significantly contributing to the increase in regulatory applications and approvals, leading to market development. For instance, in June 2024, Alembic Pharmaceuticals Ltd announced that they received the final approval from the United States health regulator for their generic Icatibant injection indicated for the treatment of acute attacks of hereditary angioedema in adults. The FDA approval is poised to bring a new and effective treatment option into the market, potentially solidifying the presence of Alembic Pharmaceuticals Ltd in the market while contributing to market growth.Rising Collaborations to Expand Accessibility for Medications in Underserved Areas
The market players are increasing efforts to expand their market presence by attempting to serve more regions other than their primary hubs. Market expansion plays a crucial role in increasing the availability of the treatment in other regions and bridging the gaps of unmet need for appropriate treatments. For instance, in June 2024, Otsuka Pharmaceutical Co., Ltd. (Otsuka) announced the expansion of the licensing agreement for hereditary angioedema (HAE) drug candidate donidalorsen (genetic name) with Ionis Pharmaceuticals, Inc. (Ionis) to widen its development and commercialisation area to the Asia-Pacific region, including Japan. Otsuka has further planned to submit applications for regulatory approval of donidalorsen in Europe and the Asia-Pacific region. The company plans to apply for regulatory approval and exclusively commercialise the product in Asian-Pacific markets. The collaboration between companies is projected to stimulate market growth by delivering donidalorsen to patients across Asia Pacific and Europe to address the unmet medical needs of patients with HAE.Hereditary Angioedema Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:
Introduction of Novel Therapies
There is a growing focus on advanced treatments such as monoclonal antibodies and gene therapies aimed at reducing attack frequency and improving long-term outcomes, bolstering market growth.Rising Awareness
The increasing awareness of the disorder is leading to an increase in the early detection of HAE by healthcare providers, creating a larger patient pool and demand for appropriate treatments.Expansion into Emerging Markets
The underserved regions with unmet needs are becoming the new focus of pharmaceutical companies. The market players are increasing efforts to expand their global presence, strengthening market development.Rising Collaborations to Offer Effective Therapeutic Solutions
Companies are increasingly entering into partnerships with other market players to boost their research and development activities by sharing their powerful expertise and resources, contributing to the introduction of innovative HAE therapies in the market.Hereditary Angioedema Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:Market Breakup by Drug Class
- C1 Esterase Inhibitor
- Selective Bradykinin B2 Receptor Antagonist
- Kallikrein Inhibitor
- Others
Market Breakup by Route of Administration
- Intravenous
- Subcutaneous
- Others
Market Breakup by End User
- Hospitals
- Clinics
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Hereditary Angioedema Market Share
Segmentation Based on Drug Class to Witness Substantial Growth
The market report offers insights into several drug classes, namely, C1 esterase inhibitors, selective bradykinin B2 receptor antagonists, Kallikrein inhibitors and others. Out of these, the C1 Esterase inhibitor drug class dominates the market share due to its established efficacy in both acute and prophylactic treatment. It reduces the frequency and severity of HAE attacks by targeting the root cause which is C1 inhibitor deficiency. It is also available in various formulations (plasma-derived and recombinant), C1 esterase inhibitors are backed by several research, and a long track record of safety and effectiveness.Subcutaneous Administration Leads Segmentation by Route of Administration
The subcutaneous route of administration leads the market share due to its convenience, improved patient compliance, and effectiveness in delivering therapies. Unlike intravenous administration, subcutaneous treatments can often be self-administered at home, reducing healthcare costs and enhancing patient comfort. This has contributed to the rise in preference for this administration method for long-term prophylactic treatments.Hereditary Angioedema Market Analysis by Region
Based on region, the market is segmented into the United States, EU-4 (Germany, France, Italy, Spain), the United Kingdom Japan and India. The regional market is dominated by the United States due to the presence of a well-established healthcare infrastructure and high awareness of the condition. The market dominance can also be attributed to the availability of advanced therapies, strong investment in research and development, and the presence of major pharmaceutical companies, bolstering the region’s leadership. The presence of a large patient pool and favourable reimbursement policies that enable individuals to access expensive treatments, resulting in increased diagnoses, propels market growth.Leading Players in the Hereditary Angioedema Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis and strategic initiatives by the leading players. The major companies in the market are as follows:Takeda Pharmaceutical Company
Takeda Pharmaceutical is a global leader based in Japan. The company has a significant presence in the HAE market, primarily through its Firazyr, which is commonly used for the treatment of acute HAE attacks in adults. Takeda also offers Takhzyro, a preventive treatment for HAE, enhancing its portfolio in hereditary angioedema management.CSL Behring
CSL Behring is a global biotechnology leader that provides a range of therapies for rare and serious diseases, including hereditary angioedema. Their HAE portfolio includes Haegarda, a C1 esterase inhibitor (human) for routine prevention of HAE attacks in adolescent and adult patients, and Berinert, for the treatment of acute abdominal, facial, or laryngeal HAE attacks in any patient group.Pharvaris N.V.
Pharvaris is a clinical-stage biopharmaceutical company focused on bringing oral therapy to patients suffering from HAE. They are pioneering the development of new oral bradykinin B2 receptor antagonists, offering an innovative approach to the treatment and prevention of HAE attacks. The company aims to replace injectable treatments with easy-to-administer pills.
BioCryst Pharmaceuticals Inc.
BioCryst Pharmaceuticals specialises in the development and marketing of novel, oral, small-molecule medicines for rare diseases like HAE. Their key product, Orladeyo (berotralstat), is an oral, once-daily therapy designed to prevent attacks in HAE patients, representing a shift toward more patient-friendly, oral therapeutic options in the market.Other key players in the market include Ionis Pharmaceuticals Inc., KalVista Pharmaceuticals Inc., Sanofi S.A., BioMarin Pharmaceutical Inc., Adverum Biotechnologies Inc., and Novartis AG., among others.
Key Questions Answered in the Hereditary Angioedema Market
- What was the hereditary angioedema market value in 2023?
- What is the hereditary angioedema market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on drug class?
- How does segmentation by route of administration influence market dynamics?
- Who are the end-users in the market?
- What are the major factors aiding the hereditary angioedema market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major hereditary angioedema market trends?
- Which drug class is expected to dominate the market?
- Which route of administration will lead the market segment?
- Which end-user will lead the market?
- Who are the key players involved in the hereditary angioedema market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Takeda Pharmaceutical Company
- CSL Behring
- Pharvaris N.V.
- BioCryst Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 3.76 Billion |
| Forecasted Market Value ( USD | $ 7.68 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |