Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive indicators, the industry confronts significant hurdles stemming from a complicated regulatory environment and restrictive reimbursement frameworks. Variable insurance coverage for thorough genomic profiling frequently limits patient access and imposes financial strains on healthcare systems. Consequently, the substantial capital investment needed to build testing infrastructure, coupled with the unpredictability of reimbursement policies, acts as a primary constraint that threatens to impede the widespread global implementation of these diagnostic solutions.
Market Drivers
The shift toward liquid biopsy for early cancer detection acts as a major driver for market growth, transforming oncology from reactive tissue sampling to proactive, non-invasive blood monitoring. This innovation allows clinicians to detect circulating tumor DNA with high sensitivity, identifying minimal residual disease and enabling personalized treatment plans without surgical risks. The necessity for such scalable diagnostics is emphasized by the rising disease burden; the American Cancer Society projected in 2025 that over 2,041,000 new cancer cases would be diagnosed in the United States alone. Leading providers are seeing significant adoption, as evidenced by Natera, Inc., which reported a 56.7% year-over-year revenue increase in its February 2025 financial results, attributed largely to the rapid volume growth of its liquid biopsy and genetic testing offerings.Simultaneously, rising government investment in population genomics is building the essential infrastructure for widespread precision medicine. National programs are funding the sequencing of large cohorts to establish diverse genomic biobanks, which accelerate drug discovery and elucidate the genetic basis of complex diseases in underrepresented groups. This government-led initiative significantly increases the volume of clinical-grade data available for research, driving demand for high-throughput sequencing. For example, the National Institutes of Health announced in February 2025 that the 'All of Us Research Program' had released whole genome sequences from over 414,000 participants, representing a nearly 70% expansion of the available dataset, thereby acting as a key engine for the global adoption of genomic testing technologies.
Market Challenges
The Global Precision Genomic Testing Market faces substantial obstacles due to reimbursement limitations and inconsistent insurance coverage, which impede the broad utilization of comprehensive genomic profiling. Although sequencing costs have decreased, the absence of standardized reimbursement policies across public and private payers creates financial instability for clinical laboratories and medical providers. Insurers frequently categorize advanced genomic tests as experimental, resulting in regular claim denials or demands for extensive prior authorization. This uncertainty deters physicians from prescribing necessary diagnostics, as they are concerned about imposing heavy out-of-pocket costs on patients or absorbing the expenses within their practice.Moreover, these administrative burdens disrupt medical practice workflows, requiring significant resources to manage complex approval procedures rather than focusing on patient care. The volatility of payment models hinders market expansion by restricting the capital available for laboratories to invest in essential testing infrastructure. According to the American Medical Association, 94% of physicians reported in 2024 that prior authorization protocols used by insurers caused delays in patient care. This statistic highlights the operational impact of reimbursement barriers, which directly limits patient access to precision medicine and curtails the overall growth potential of the market.
Market Trends
The incorporation of Artificial Intelligence (AI) and Machine Learning (ML) is transforming the market by automating complex variant interpretation and facilitating the analysis of extensive multi-modal datasets. As genomic sequencing yields immense volumes of data, AI algorithms address bottlenecks in traditional bioinformatics pipelines by identifying pathogenic variants with enhanced speed and precision. This technological advancement is fueling commercial growth for companies utilizing AI-driven clinical data platforms; for instance, Tempus AI reported in February 2025 that its Data and Services segment achieved $241.6 million in annual revenue, a 43.2% year-over-year increase that underscores the growing value of AI-enabled genomic insights.Additionally, the rise of companion diagnostic (CDx) collaborations is accelerating as pharmaceutical developers increasingly depend on precision testing to direct targeted therapies. This trend involves diagnostic providers partnering with biopharmaceutical companies during early-stage clinical trials to validate comprehensive genomic profiling assays alongside new drugs, rather than retrospectively. These strategic alliances significantly boost test volumes linked to drug development and trial enrollment, establishing a consistent revenue stream for providers. In January 2025, Guardant Health reported a 35% year-over-year increase in biopharma test volumes, totaling approximately 40,500 tests, driven by partner demand for its precision oncology solutions.
Key Players Profiled in the Precision Genomic Testing Market
- Danaher Corporation
- Merck KGaA
- Revvity, Inc.
- Maravai LifeSciences
- GenScript Biotech
- QIAGEN N.V.
- PacBio Biosciences Inc.
- Oxford Nanopore Technologies plc
- Illumina, Inc.
- 10x Genomics, Inc.
Report Scope
In this report, the Global Precision Genomic Testing Market has been segmented into the following categories:Precision Genomic Testing Market, by Technology:
- Next-Generation Sequencing
- Polymerase Chain Reaction
- Microarray Technology
- Sanger Sequencing
- CRISPR/Cas Systems
- Others
Precision Genomic Testing Market, by Product & Services:
- Consumables (Kits & Reagents)
- Equipment
- Services
Precision Genomic Testing Market, by Application:
- Oncology
- Cardiovascular Diseases
- Neurological Disorders
- Reproductive Health
- Rare Diseases
- Others
Precision Genomic Testing Market, by End-Use:
- Hospitals and Clinics
- Diagnostic Laboratories
- Research & Academic Institutes
- Others
Precision Genomic Testing Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Precision Genomic Testing Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Precision Genomic Testing market report include:- Danaher Corporation
- Merck KGaA
- Revvity, Inc.
- Maravai LifeSciences
- GenScript Biotech
- QIAGEN N.V.
- PacBio Biosciences Inc.
- Oxford Nanopore Technologies PLC.
- Illumina, Inc.
- 10x Genomics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
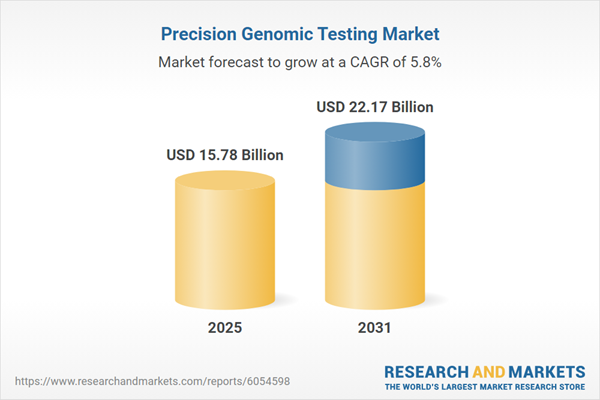
| Estimated Market Value ( USD | $ 15.78 Billion |
| Forecasted Market Value ( USD | $ 22.17 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |