Speak directly to the analyst to clarify any post sales queries you may have.
The at-home urine testing market is evolving rapidly, driven by enhanced consumer demand for accessible health insights and the pursuit of patient-centric diagnostic solutions. Senior decision-makers face critical opportunities to align with dynamic trends, optimize supply chains, and deliver differentiated value within this expanding landscape.
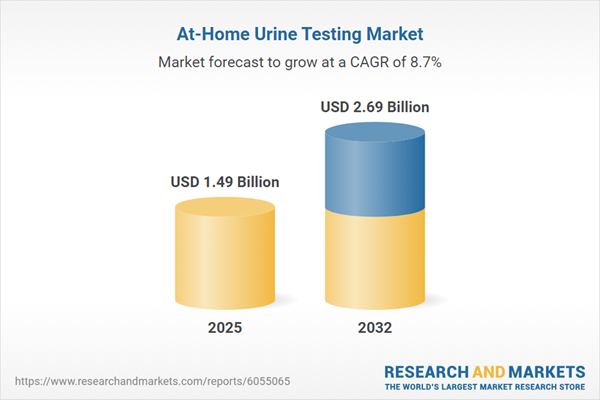
Market Snapshot: At-Home Urine Testing Market Growth and Projections
The global at-home urine testing market grew from USD 1.38 billion in 2024 to USD 1.49 billion in 2025, and is projected to continue rising at a compound annual growth rate (CAGR) of 8.68%, reaching USD 2.69 billion by 2032. This robust growth underscores a shift toward decentralized, patient-managed diagnostics. Enhanced adoption rates reflect increasing awareness of chronic health conditions, wider self-monitoring, and broader telehealth integration. Industry leaders are capitalizing on sustained demand through innovation, regulatory alignment, and digital transformation to expand their reach in key markets worldwide.
Scope & Segmentation
- Product Types: Urine collection devices, urine test kits, and a broad assortment of urine test strips, including those for drugs, glucose, ketones, pH, pregnancy, and protein.
- Technology: Bioanalytical sensors, colorimetry-based testing systems, digital urine testers, enzyme-linked immunosorbent assay (ELISA) platforms, and immunochromatographic lateral flow assays.
- Collection Methods: First-morning, midstream, and timed urine sampling—each influencing result accuracy and user experience.
- Product Forms: Multi-use analyzers and single-use testing products to serve both frequent users and occasional testers.
- Distribution Channels: Offline points such as pharmacies and supermarkets, along with online direct-to-consumer stores and third-party e-commerce platforms.
- Applications: Diabetes monitoring, drug and alcohol testing, kidney function checks, obesity and ketosis assessment, pharmacy and wellness panels, pregnancy, and UTI diagnostics.
- Geographies Covered: Regional analysis spans the Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), EMEA (including major European, Middle Eastern, and African countries), and Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
Key Takeaways for Senior Decision-Makers
- At-home urine testing is central to the decentralization of diagnostics, empowering both patients and providers to proactively manage health from home.
- Technology advances in sensors, colorimetric and immunochromatographic assays are narrowing the quality gap between home testing and centralized laboratory solutions.
- The integration with telehealth and mobile health applications creates actionable, real-time feedback loops, driving patient engagement and adherence.
- Segment growth is particularly robust in drug screening, diabetes management, and multi-panel wellness tracking, driven by rising consumer demand for actionable convenience.
- Supply chain adaptability and strategic procurement are essential in managing cost volatility related to tariffs and global sourcing challenges.
Tariff Impact: Navigating US Trade Policy Shifts
The implementation of United States tariffs in 2025 introduces increased input costs across raw materials and finished goods. Industry leaders are responding by reassessing sourcing strategies, diversifying suppliers, and, where feasible, emphasizing domestic production. These adaptations support margin stability and competitive positioning as companies mitigate risks associated with logistics delays, higher freight expenses, and fluctuating duty structures. Multinational firms are leveraging vertical integration and long-term vendor relationships to buffer operational challenges in this new regulatory environment.
Methodology & Data Sources
This research utilizes a blended approach that combines an extensive review of peer-reviewed literature, regulatory filings, patent databases, and company disclosures with direct interviews from industry stakeholders. Triangulation across primary and secondary sources ensures robust validation and accuracy, while thorough quality checks underpin all findings.
Why This Report Matters
- Supports strategic planning by illuminating drivers, inhibitors, and opportunities across product, technology, channel, and regional segments.
- Enables informed investment and supply chain decisions through analysis of regulatory and trade impacts, particularly tariff-sensitive environments.
- Equips senior leaders with actionable insights that guide market entry, expansion, and innovation roadmaps tailored to the evolving at-home urine testing sector.
Conclusion
The at-home urine testing market is positioned for ongoing growth as digital integration, patient empowerment, and technological innovation converge. Senior leaders, equipped with clear insight, are well-positioned to optimize strategies and capture emerging opportunities in this dynamic healthcare segment.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this At-Home Urine Testing market report include:- Abbott Laboratories
- ACON Laboratories, Inc.
- AdvaCare Pharma
- AlphaBiolabs Ltd.
- AZO Products
- Church & Dwight Co., Inc.
- Clinical Guard
- Easy Healthcare Corporation
- Equinox Biotech Co., Ltd.
- Everlywell, Inc.
- Laboratory Corporation of America Holdings
- LIFE2O
- Mankind Pharma Limited
- Medline Industries, Inc.
- MomMed
- myLAB Box
- PREGMATE
- Quest Diagnostics Incorporated
- Roman Health Ventures Inc.
- Siemens Healthineers AG
- SPD Swiss Precision Diagnostics GmbH
- Target Brands, Inc.
- Trividia Health, Inc.
- Viatris Inc.
- Walgreens Boots Alliance, Inc.
- Walmart Inc.
- Winx Health, Inc.
- ZRT Laboratory LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.49 Billion |
| Forecasted Market Value ( USD | $ 2.69 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |