Speak directly to the analyst to clarify any post sales queries you may have.
Perfusion bioreactors are redefining modern biomanufacturing approaches by providing organizations with new levels of efficiency, flexibility, and process reliability, helping senior decision-makers adapt to increasing production demands and operational complexity.
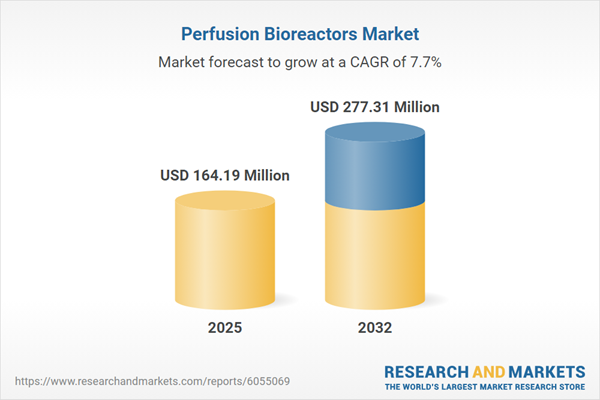
Market Snapshot: Growth Trajectory of the Perfusion Bioreactors Market
The Perfusion Bioreactors Market continues to expand robustly, supported by a strong current valuation and a healthy compound annual growth rate (CAGR) projected through 2032. Continuous bioprocessing methods are garnering increased confidence, especially as the sector seeks to deliver next-generation biologics and sophisticated therapies. With mounting regulatory expectations and technological advances, the need for precision and repeatability is intensifying. Pharmaceutical manufacturers, contract manufacturing organizations, and biotechnology innovators are refining integration strategies to excel in a regulated, rapidly transforming market landscape.
Scope & Segmentation of the Perfusion Bioreactors Market
This report illuminates the key drivers, segments, and technological trends advancing the Perfusion Bioreactors Market and influencing organizational decision-making:
- Product Types: Choice between multi-use bioreactors, which bolster reliability for established, large-scale operations, and single-use systems, offering agility for projects that require frequent changes and rapid turnaround.
- Culture Types: Solutions support animal, microbial, and plant cell cultures—each demanding tailored system adaptations for optimal cell health and consistent product quality.
- Modes of Operation: Batch, fed-batch, and continuous perfusion models are available, enabling organizations to align production mechanisms with throughput goals, automation requirements, and regulatory compliance.
- Capacities: Scale extends from compact, research-oriented units under 10 liters, up to commercial-grade systems exceeding 100 liters, providing seamless growth across research and manufacturing phases.
- Material Types: Glass constructions allow superior observation and analytical capabilities, plastics drive single-use process innovation and stringent contamination control, while stainless steel offers enduring performance for intensive manufacturing lines.
- Applications: Covers gene therapy, stem cell therapy, monoclonal antibody development, vaccine production, and recombinant protein manufacturing, underlining a wide span of advanced therapeutic development use cases.
- End Users: Includes biopharmaceutical enterprises, contract manufacturing service providers, biotech innovators, and research institutes—each seeking to leverage bioreactor solutions for higher yield, scalability, and compliance.
- Regions: North America and Asia-Pacific continue to drive leading adoption, while Europe, Latin America, the Middle East, and Africa contribute unique opportunities and regulatory considerations shaped by infrastructure and local partnership expansion.
- Leading Companies Covered: CELLEC BIOTEK AG, INFROS HT, 3D Biotek LLC, bbi-biotech GmbH, Cell Culture Company LLC., Colder Products Company, Cytiva by Danaher Corporation, FiberCell Systems Inc, Merck KGaA, Nanjing BioPAS Pharmaceutical Equipment Co., PBS Biotech Inc., Sartorius AG, SYNTHECON INCORPORATED, TA Instruments, Zellwerk GmbH.
Key Takeaways: Strategic Insights for Decision-Makers
- Deployment of advanced sensors and digital control platforms is standardizing response times, enhancing process transparency, and supporting compliance for organizations managing regulated production workflows.
- Leaders are increasingly evaluating the trade-offs between multi-use and single-use bioreactors to maximize project responsiveness, regulatory alignment, and return on capital invested.
- Development of specialized bioreactor designs reflects a push to address unique cell culture requirements, boosting product integrity and process yields for both research and clinical-grade manufacturing.
- Introduction of continuous production models, alongside modular configurations, substantially reduces manual intervention and contamination risk, thereby improving consistency and operational scalability.
- Cell retention innovations and configurable architectures ease downstream integration, helping organizations address a diverse and evolving biotherapeutic pipeline with agility.
- North America and Asia-Pacific set the pace for technology adoption, while other regions focus on strengthening partnerships, adapting regulatory frameworks, and building capabilities for bioreactor integration.
Tariff Impact: Navigating Changing Cost Structures
New tariffs imposed by the United States in 2025 have increased expenditure on essential imported bioreactor components, including pumps, sensors, and consumables. In response, manufacturers are intensifying local production, forming more robust domestic supply partnerships, and combining custom assembly with standardized parts. These strategies are improving supply chain durability and helping insulate biomanufacturing operations from future trade uncertainties.
Methodology & Data Sources
This market analysis is informed by a combination of direct stakeholder interviews, rigorous research standards, and scrutiny of secondary materials, such as regulatory records and sector publications. Insights are validated through data triangulation and SWOT analysis for increased credibility and forecasting accuracy.
Why This Report Matters for Senior Decision-Makers
- Extensive segmentation and in-depth regional perspectives offer actionable intelligence for targeting investments and optimizing market entry timing.
- Supports executive teams in managing operational risk, aligning resources, and responding to shifting regulatory demands and technological advances across geographies.
- Includes benchmarking resources for evaluating supplier competencies and ensuring organizational standards meet evolving industry performance metrics.
Conclusion
Innovation in perfusion bioreactors is equipping organizations to better match growth in biomanufacturing needs with improved flexibility and operational strength. Early system adoption will empower leaders to achieve greater resilience within increasingly complex industry conditions.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Perfusion Bioreactors Market report include:- CELLEC BIOTEK AG
- INFROS HT
- 3D Biotek LLC
- bbi-biotech GmbH
- Cell Culture Company, LLC.
- Colder Products Company
- Cytiva by Danaher Corporation
- FiberCell Systems Inc
- Merck KGaA
- Nanjing BioPAS Pharmaceutical Equipment Co.
- PBS Biotech, Inc.
- Sartorius AG
- SYNTHECON, INCORPORATED
- TA Instruments
- Zellwerk GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 164.19 Million |
| Forecasted Market Value ( USD | $ 277.31 Million |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |