Speak directly to the analyst to clarify any post sales queries you may have.
The cardiopulmonary support system market stands at the forefront of innovation in clinical care, evolving rapidly to address increasingly complex patient needs. Senior decision-makers require in-depth, actionable intelligence to navigate technological shifts, regulatory complexity, and competitive landscapes shaping the future of cardiopulmonary support solutions.
Market Snapshot: Cardiopulmonary Support System Market
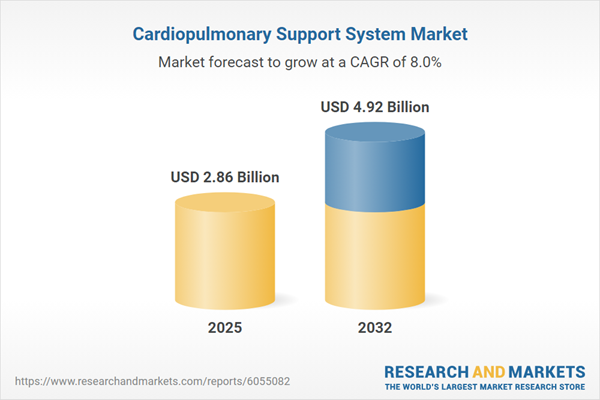
The Cardiopulmonary Support System Market grew from USD 2.65 billion in 2024 to USD 2.86 billion in 2025. It is expected to continue growing at a CAGR of 8.02%, reaching USD 4.92 billion by 2032.
This robust trajectory reflects rising clinical demand for advanced life-support technologies in acute and chronic environments, fueled by demographic shifts and increasing global disease prevalence.
Scope & Segmentation
This report offers a comprehensive analysis, segmenting the cardiopulmonary support system market by product category, application, patient type, end use, and region. Major industry participants and transformative technologies are featured for granular insight.
- Product Type: Circulation support devices, consumables, ECMO systems, oxygenators, and ventilators.
- Application: Cardiac and respiratory support, addressing emergent cardiac dysfunction, post-cardiotomy care, and chronic respiratory exacerbations.
- Patient Type: Geriatric and pediatric populations, with specific solutions tailored for comorbidities and anatomical nuances.
- End Use: Hospitals, ambulatory surgical centers, and specialty clinics employing varying service models and workflows.
- Region: Americas, Europe, Middle East, Africa, and Asia-Pacific, each shaped by healthcare infrastructure maturity, regulatory environment, and economic drivers.
- Company Coverage: Profiles leading firms such as Abbott Laboratories, Boston Scientific Corporation, Medtronic PLC, Siemens AG, and others engaged in ongoing product and service innovation.
Key Takeaways for Decision-Makers
- Growing prevalence of chronic cardiovascular and respiratory conditions is intensifying the need for advanced, adaptable support systems across care settings.
- Technological advancements, including the miniaturization of pumps and integration of biocompatible membranes, improve device portability and enable long-term, out-of-hospital use.
- Digital health platforms and interoperable monitoring systems offer enhanced data analytics, real-time alerting, and support multidisciplinary clinical decision-making.
- Tailored device offerings for specific patient demographics, especially pediatric and elderly populations, expand market access and improve outcomes through personalized solutions.
- Collaborative ecosystems involving manufacturers, regulators, and providers are streamlining innovation adoption and reinforcing post-market safety standards.
Tariff Impact and Market Adaptation
The implementation of increased tariffs in 2025 added pressure to global supply chains for cardiopulmonary support equipment. Manufacturers responded by reassessing sourcing strategies, exploring nearshoring, and establishing regional assembly operations to reduce reliance on overseas shipments. Cost-sharing agreements and inventory management initiatives helped healthcare providers adapt despite acquisition challenges, particularly among smaller clinics. These approaches underscore the sector’s focus on supply chain resilience and agile procurement practices.
Methodology & Data Sources
This report combines rigorous secondary research with targeted primary interviews. Leading cardiothoracic surgeons, biomedical engineers, and procurement executives contributed to market validation and scenario development. Qualitative findings were cross-checked against industry utilization trends, while regional policy and infrastructure assessments informed the analysis.
Why This Report Matters
- Offers clear guidance for portfolio and investment decisions based on current adoption trends and projected demand drivers.
- Identifies regional opportunities and regulatory nuances, equipping leaders to adapt strategies for effective market expansion.
- Supports informed procurement, supply chain planning, and partnership development through actionable segment analysis and benchmarking.
Conclusion
Market leaders in cardiopulmonary support are leveraging digital integration and patient-centric innovation to reshape clinical care. Future success depends on informed strategies, robust supplier networks, and agile regulatory engagement in this evolving landscape.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Cardiopulmonary Support System Market report include:- Abbott Laboratories
- Baxter International
- BIOTRONIK SE & Co KG
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Eurosets S.r.l
- Fresenius Medical Care AG
- General Electric Company
- Getinge AB
- Jarvik Heart, Inc.
- Johnson & Johnson Services, Inc.
- LivaNova PLC
- Medtronic PLC
- MicroPort Scientific Corporation
- Nipro Corporation
- Penumbra, Inc.
- Senko Medical Instrument Manufacturing Co., Ltd.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Siemens AG
- Spectrum Medical Ltd
- Stryker Corporation
- SynCardia Systems, LLC
- Terumo Corporation
- Lepu Medical Technology(Beijing)Co.,Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.86 Billion |
| Forecasted Market Value ( USD | $ 4.92 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |