Speak directly to the analyst to clarify any post sales queries you may have.
The Chylomicronemia Syndrome Market is experiencing meaningful transformation as scientific advances drive new diagnostic and therapeutic possibilities, from molecular genetics to precision care delivery. This environment demands agile decision-making and strategic foresight from senior executives navigating evolving regulatory and market dynamics.
Market Snapshot: Navigating the Chylomicronemia Syndrome Market
The market for chylomicronemia syndrome demonstrates steady growth, supported by a combination of technology investments and clinical innovation. Emerging tools in genetic testing and lipid profiling have advanced the accuracy and reach of diagnostics, while a pipeline of novel therapies expands the potential for enduring disease management. Stakeholders are responding to clinical, regulatory, and supply chain complexities to align business models with evolving healthcare needs. The industry’s outlook is shaped by adaptive product development, tariff-influenced sourcing, and increasing payer engagement on both sides of the Atlantic and beyond.
Scope & Segmentation of the Chylomicronemia Syndrome Market
This report offers senior leaders comprehensive visibility into critical market segments and technological advancements:
- Product Portfolio: Diagnostics tools (genetic testing, lipid profile, ultrasound), supplements, and treatments (dietary modifications, gene therapy, plasma exchange)
- Treatment Types: Dietary management, various pharmacotherapy options (fibrates, niacin, novel agents, omega-3 fatty acids, statins), gene therapy, and plasmapheresis
- Diagnostic Methods: Genetic testing and imaging techniques are central for early detection, clinical monitoring, and personalized assessment
- Applications: Research and development, routine screening, and treatment facilitation, reflecting the pathway from innovation to practical care delivery
- End-Users: Contract research organizations, diagnostic laboratories, hospitals, and research institutes, signifying diverse involvement from discovery to end care
- Distribution Channels: Hospital pharmacies, online pharmacies, retail pharmacies, and specialty outlets, providing access through traditional and digital pathways
- Regional Markets: Analysis covers the Americas, Europe, Middle East, Africa, and Asia-Pacific, addressing regional nuances in regulation, technology adoption, and patient access
- Key Players: Profiles of companies such as Abbott Laboratories, Ionis Pharmaceuticals, Quest Diagnostics, Regeneron Pharmaceuticals, and others driving current and future progress
Key Takeaways for Executives
- Market stakeholders must proactively address clinical heterogeneity and complex treatment pathways through cross-functional, integrated strategies.
- Advancements in genetic sequencing, antisense oligonucleotides, and gene therapies are moving the market beyond traditional dietary and pharmacological approaches.
- Evolving payer policies and regional guidelines increasingly prioritize personalized medicine and early intervention, creating demand for robust health economics evidence.
- Collaborative partnerships—spanning academic centers, biopharma, and diagnostic innovators—are enhancing technology transfer and pipeline progression.
- Supply chain disruptions and regulatory shifts, especially those related to tariffs, have prompted companies to pivot toward local production and strategic sourcing partnerships.
Tariff Impact: Strategic Supply Chain Adjustments
The introduction of tariffs on medical devices and therapeutic inputs in the United States has elevated procurement costs across diagnostics and therapy development. Stakeholders, including contract research organizations and specialty pharmacies, are recalibrating sourcing, emphasizing regional manufacturing, and adjusting inventory strategy to mitigate delays and unexpected price escalations. These interventions are generating new industry collaborations to facilitate regulatory compliance and ensure continuity of patient care amid shifting trade policies.
Methodology & Data Sources
The analysis integrates secondary research from peer-reviewed literature, regulatory filings, and industry reports with primary expert interviews and consensus-driven forecasting. Reliability is enhanced through triangulation, peer review, and advanced analytical frameworks to capture competitor moves, regulatory influences, and scientific advancement.
Why This Report Matters
- Enables executive teams to align market expansion initiatives with evolving diagnostic and therapeutic innovation, supporting long-term value creation.
- Offers actionable insight into the impact of global regulatory and economic changes—particularly tariffs—on sourcing, pricing, and patient access strategies.
- Equips organizations to benchmark progress, identify partnership opportunities, and support resilient supply chains for enhanced operational readiness.
Conclusion
As the Chylomicronemia Syndrome Market advances, informed strategic alignment and operational agility position decision-makers for success across the clinical and commercial spectrum. Timely insight and proactive adaptation remain vital to sustainable growth and competitive differentiation.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Chylomicronemia Syndrome market report include:- Abbott Laboratories
- Aegerion Pharmaceuticals
- Alnylam Pharmaceuticals, Inc.
- Arrowhead Pharmaceuticals
- Bluebird Bio
- Editas Medicine
- Esperion Therapeutics, Inc.
- Intellia Therapeutics
- Ionis Pharmaceuticals, Inc.
- Laboratory Corporation of America Holdings
- Novo Nordisk A/S
- Quest Diagnostics
- Regeneron Pharmaceuticals, Inc.
- Scribe Therapeutics
- Visirna Therapeutics HK Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
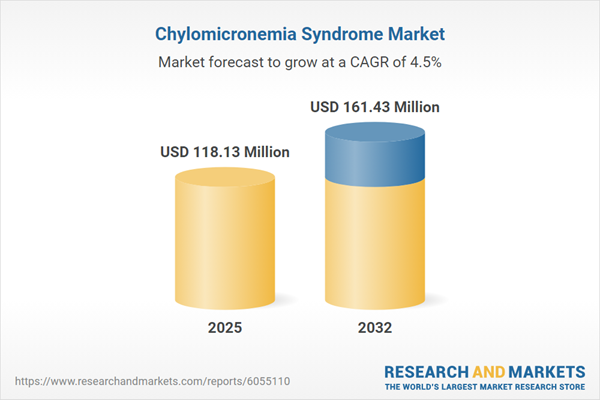
| Estimated Market Value ( USD | $ 118.13 Million |
| Forecasted Market Value ( USD | $ 161.43 Million |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |