Speak directly to the analyst to clarify any post sales queries you may have.
Discover How Pulsed-Field Ablation is Redefining Cardiac Arrhythmia Management with Advanced Nonthermal Technology Delivering Precision and Safety
Pulsed-field ablation has emerged as a disruptive force in the management of cardiac arrhythmias, offering a novel, nonthermal modality that significantly reduces collateral tissue damage. Unlike traditional thermal techniques, this approach harnesses ultra-short electrical pulses to create targeted lesions within myocardial tissue, preserving surrounding structures and streamlining procedural workflows. As the clinical community seeks safer, more efficient therapies, pulsed-field ablation has garnered attention for its precision, rapid lesion formation, and favorable safety profile.Moreover, the evolution of electrophysiology laboratories toward highly integrated, data-driven environments has accelerated adoption. Real-time imaging integration, advanced mapping systems, and adaptive energy delivery algorithms converge to deliver outcomes that rival-and in some cases surpass-established ablation modalities. This convergence lays the groundwork for broader clinical acceptance and reimbursement support, addressing key barriers to market penetration. Transitioning from concept to standard of care, pulsed-field ablation represents both a technological breakthrough and a strategic inflection point for device manufacturers, healthcare providers, and payers.
Ultimately, this introduction frames pulsed-field ablation not merely as an incremental improvement but as a transformative therapy poised to redefine benchmarks for safety, efficacy, and operational efficiency in electrophysiology.
Unveiling the Technological and Clinical Milestones that Have Transformed Pulsed-Field Ablation into a Forefront Cardiac Therapy with Future Growth Trajectories
Over the past decade, pivotal clinical trials and regulatory approvals have catalyzed a significant shift in the landscape of cardiac ablation. The introduction of purpose-built catheters capable of delivering high-voltage, short-duration pulses has propelled pulsed-field ablation from early-stage research into mainstream practice. Concurrently, refinements in waveform delivery and catheter design have enhanced lesion durability while minimizing procedural complications. These technological milestones have, in turn, influenced protocol development, driving consistency in outcomes across diverse patient populations.In parallel, investment from leading cardiovascular device companies has spurred collaborative initiatives, merging engineering expertise with clinical insights. The result is a convergence of best practices that elevates procedural predictability and operational efficiency. Furthermore, the integration of sophisticated mapping software has transformed pre-procedural planning, enabling electrophysiologists to tailor energy delivery with unprecedented accuracy. Consequently, clinics are witnessing shorter procedure times and more efficient resource utilization, reinforcing the value proposition of pulsed-field ablation.
Taken together, these advances underscore a broader trend toward personalized, image-guided therapy in electrophysiology, illustrating how collaborative innovation can reshape therapeutic paradigms and position pulsed-field ablation at the forefront of cardiac intervention.
Analyzing the Comprehensive Effects of United States Tariff Implementations in 2025 on Pulsed-Field Ablation Equipment Supply Chains and Cost Structures
The advent of new tariff structures in 2025 significantly impacts the import and distribution of pulsed-field ablation equipment. Manufacturers reliant on cross-border supply chains for generators, catheters, and specialized components now face increased cost pressures that reverberate across the value chain. Equipment suppliers are reevaluating sourcing strategies, often shifting production closer to end markets to mitigate tariff burdens. In doing so, they balance the trade-off between supply chain resilience and manufacturing costs.Hospitals and ambulatory surgical centers must navigate these changes in procurement practices. With higher landed costs, end users are engaging in more rigorous cost-benefit analyses, weighing upfront capital investments against projected gains in procedural efficiency and patient outcomes. Simultaneously, distributors are restructuring pricing models to absorb some of the tariff-related cost increases, preserving competitive positioning in key regions. This restructuring often involves negotiating long-term contracts that provide price stability and predictable delivery schedules.
Ultimately, the ripple effect of US tariffs extends beyond pricing, influencing strategic decisions around clinical adoption, inventory management, and partnership models. Providers and manufacturers alike must adapt by forging more collaborative relationships and exploring innovative financing mechanisms to align economic realities with clinical imperatives.
Decoding Segmentation Insights Revealing How Device Components, Clinical Indications, and End-User Profiles Drive Pulsed-Field Ablation Adoption Dynamics
Segmentation analysis reveals how component offerings, clinical indications, and end-user profiles shape pulsed-field ablation adoption. The component breakdown distinguishes between devices and the supporting software that enables real-time visualization and energy delivery optimization. Within the devices category, catheters and generators serve as the cornerstone of procedural efficacy, directly influencing lesion quality and consistency.Similarly, the clinical landscape diverges based on indication, with atrial fibrillation ablation drawing significant focus due to its prevalence and the demand for minimally invasive solutions. In parallel, supraventricular tachycardia ablation continues to benefit from pulsed-field approaches, particularly in patient subsets where thermal methods pose higher risks. This dual-indication framework underscores the adaptability of pulsed-field systems across diverse arrhythmogenic substrates.
The end-user segmentation further elucidates adoption dynamics, highlighting differences between ambulatory surgical centers that prioritize procedural throughput and cost efficiency, and hospitals and clinics that emphasize comprehensive care pathways and integration with existing electrophysiology infrastructure. Understanding these distinct requirements is essential for manufacturers seeking to tailor their product portfolios, service models, and marketing strategies to maximize clinical and economic value.
Illuminating Key Regional Market Dynamics Across Americas, Europe Middle East & Africa, and Asia-Pacific Fueling Growth in Pulsed-Field Ablation Technologies
Regional analysis highlights divergent growth drivers and market characteristics across the Americas, Europe Middle East & Africa, and Asia-Pacific. In the Americas, early clinical adoption is supported by a robust reimbursement environment and a concentration of high-volume electrophysiology centers. This region benefits from rapid regulatory pathways and strong partnerships between device innovators and leading academic institutions, facilitating real-world evidence generation and early market validation.Across Europe, Middle East & Africa, regulatory harmonization efforts and pan-regional reimbursement frameworks have lowered barriers to entry for pulsed-field technologies. Investment in specialized cardiac centers and cross-border clinical collaborations accelerates knowledge sharing. However, localized pricing pressures and varying healthcare infrastructure maturity necessitate tailored commercialization strategies that balance cost-effectiveness with clinical differentiation.
In Asia-Pacific, expanding healthcare access and rising investments in cardiovascular care are primary growth catalysts. Governments and private entities alike are prioritizing minimally invasive procedures to address the growing burden of arrhythmias. While regulatory timelines may vary, strategic alliances between global device companies and local partners have proven instrumental in navigating market entry complexities and scaling adoption across diverse healthcare ecosystems.
Highlighting Strategic Moves and Innovations from Leading Industry Participants Shaping the Competitive Pulsed-Field Ablation Market Landscape Worldwide
Leading medical device companies are driving innovation and shaping the competitive landscape of pulsed-field ablation. Boston Scientific has leveraged its acquisition of a pioneering pulsed-field platform to expand its electrophysiology portfolio, integrating advanced mapping capabilities with proprietary generator technology. Similarly, a major Johnson & Johnson subsidiary has focused on optimizing catheter designs to improve maneuverability and lesion precision, investing in head-to-head clinical studies that validate safety outcomes.Medtronic continues to refine waveform algorithms and reinforce strategic partnerships with academic centers, enhancing its global market reach through collaborative data-sharing initiatives. Meanwhile, emerging players such as AtriCure are strategically positioning themselves by emphasizing niche applications and building specialized service networks that support training and procedural efficiency. Startup ventures have also attracted venture capital interest, advancing next-generation catheter materials and miniaturized generator systems with the potential to further reduce procedural complexity.
Collectively, these companies are not only advancing product innovation but also redefining value propositions through bundled service offerings, subscription-based software licenses, and outcome-linked contracting models. Their combined efforts are accelerating technology maturation and setting new benchmarks for safety, efficacy, and cost-effectiveness in pulsed-field ablation.
Empowering Industry Leaders with Targeted Strategies to Accelerate Adoption, Enhance Efficiency, and Capitalize on Opportunities in Pulsed-Field Ablation
Industry leaders should proactively invest in cross-functional collaboration to bridge engineering innovation with clinical practice, ensuring that product roadmaps align with evolving electrophysiology workflows. Establishing dedicated centers of excellence fosters early adoption, accelerates clinician training, and generates robust real-world evidence, which in turn strengthens payer negotiations and reimbursement support.Manufacturers must also explore modular technology architectures that allow incremental upgrades to software and hardware, reducing the total cost of ownership and prolonging the operational lifespan of installed base equipment. This approach not only enhances customer loyalty but also creates recurring revenue streams that can buffer against pricing pressures and tariff fluctuations.
Collaborating with healthcare providers to design value-based contracting models tied to clinical outcomes will differentiate product offerings and mitigate adoption barriers. By sharing risk through performance guarantees or milestone-based pricing, vendors can demonstrate commitment to patient safety and procedural efficacy, ultimately driving preference for pulsed-field systems over legacy thermal platforms.
Finally, strengthening global supply chain resilience through strategic dual sourcing and nearshoring initiatives will minimize disruptions related to regulatory changes and trade policies. By diversifying manufacturing footprints and forging partnerships with local contract manufacturers, companies can maintain competitive pricing while ensuring timely delivery of critical components.
In-Depth Overview of Research Methodology Combining Rigorous Primary Interviews, Comprehensive Secondary Data Analysis, and Robust Data Triangulation
This research integrates insights from extensive primary and secondary data collection to ensure a comprehensive and balanced analysis. Primary research involved direct interviews with leading electrophysiologists, hospital procurement directors, device engineers, and regulatory specialists. These interviews provided nuanced perspectives on clinical workflows, adoption barriers, and emerging technology requirements, grounding the findings in real-world practice.Secondary research encompassed a thorough review of peer-reviewed journals, regulatory dossiers, patent filings, company white papers, and conference proceedings. This was supplemented by analysis of public financial reports and strategic investor presentations to capture the competitive intelligence and investment trends shaping the pulsed-field ablation space.
To validate conclusions, the study employed data triangulation techniques, cross-referencing insights from multiple sources to ensure consistency and reliability. Market segmentation and regional trends were further corroborated through quantitative surveys of end users across ambulatory surgical centers and hospital electrophysiology units. This mixed-methods approach reinforces the credibility of the report's strategic recommendations and ensures the findings reflect both macro-level dynamics and micro-level operational realities.
Summarizing the Strategic Imperatives and Emerging Opportunities That Define the Future Trajectory of Pulsed-Field Ablation in Cardiac Electrophysiology
In summary, pulsed-field ablation stands at the cusp of mainstream adoption, propelled by technological breakthroughs, strategic collaborations, and evolving clinical guidelines. The convergence of advanced catheter designs, sophisticated mapping software, and flexible energy delivery systems has created a compelling value proposition centered on safety, efficiency, and patient outcomes.Regional dynamics further highlight the importance of tailored market approaches, with each geography presenting distinct regulatory, economic, and clinical landscapes. Simultaneously, tariff-induced cost pressures underscore the need for resilient supply chains and innovative contracting models that align stakeholder interests and support sustainable growth.
As the competitive environment intensifies, success will hinge on the ability of industry players to integrate clinical insights with agile product development, forge strong partnerships with providers, and leverage outcome-based agreements. These strategic imperatives will define the next chapter in pulsed-field ablation and shape its impact on the broader cardiac electrophysiology field.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Component
- Devices
- Catheters
- Generators
- Software
- Devices
- Indication
- Atrial Fibrillation Ablation
- Supraventricular Tachycardia Ablation
- End User
- Ambulatory Surgical Centers
- Hospitals & Clinics
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Abbott Laboratories
- Acutus Medical, Inc.
- Boston Scientific Corporation
- CardioFocus, Inc.
- Kardium Inc.
- Medical Device Business Services, Inc
- Medtronic plc
- MicroPort Scientific Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Pulsed-Field Ablation System market report include:- Abbott Laboratories
- Acutus Medical, Inc.
- Boston Scientific Corporation
- CardioFocus, Inc.
- Kardium Inc.
- Medical Device Business Services, Inc
- Medtronic plc
- MicroPort Scientific Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
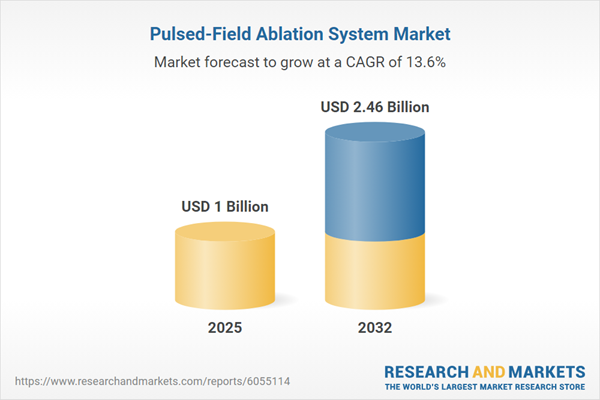
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 2.46 Billion |
| Compound Annual Growth Rate | 13.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |