Speak directly to the analyst to clarify any post sales queries you may have.
Palm vein biometrics emerges as a high-assurance, low-friction identity layer as security demands rise across physical access and digital verification
Palm vein biometrics has moved from a niche modality to a serious contender in high-assurance identity verification because it combines strong spoof resistance with user-friendly interaction. By reading the unique vascular patterns inside the hand using near-infrared illumination, it reduces dependence on surface features that are more exposed to environmental wear or direct replication attempts. This intrinsic advantage aligns with today’s authentication challenge: deliver stronger security without adding friction that erodes adoption.The technology’s relevance is also rising as organizations modernize identity stacks across physical and digital domains. Enterprises are converging building access, workforce time and attendance, privileged system access, and customer onboarding into more unified identity experiences. In that convergence, palm vein offers a compelling mix of hygienic operation options, fast matching performance, and broad acceptance in contexts where fingerprints may be culturally sensitive or operationally unreliable.
At the same time, decision-makers face practical questions that determine success: sensor selection, integration with IAM and access-control platforms, liveness detection approaches, template protection, and lifecycle governance. This executive summary frames the landscape through shifts in technology and regulation, supply chain and tariff considerations, segmentation and regional dynamics, leading company approaches, and actions leaders can take to deploy palm vein biometrics responsibly and at scale.
A new era of zero-trust identity, edge processing, and privacy governance is redefining how palm vein biometrics is evaluated and deployed
The most transformative shift is the repositioning of palm vein biometrics from “alternative biometric” to “strategic modality” in zero-trust programs. As organizations adopt continuous verification principles, they are looking beyond single-factor credentials toward layered approaches that combine possession, knowledge, and inherence. Palm vein is increasingly evaluated not only as a standalone method but as part of multimodal stacks that can adapt to context, risk level, and user population.Another major change is the rapid improvement of sensor miniaturization and embedded processing. Vendors are optimizing illumination, imaging, and feature extraction to run efficiently on edge devices, which reduces latency and can support privacy-preserving architectures where sensitive biometric processing stays local. This is complemented by better interoperability patterns through SDKs, APIs, and middleware that connect palm vein engines to identity providers, access control systems, and mobile onboarding workflows.
Threat models have also evolved. Attackers increasingly target the entire identity pipeline-enrollment, template storage, matching, and recovery-rather than only the front-end capture. As a result, buyers are demanding stronger template protection, hardware-rooted security, and auditability. Concurrently, regulatory expectations for consent, purpose limitation, and data minimization are tightening, pushing providers to offer clearer governance controls, retention policies, and privacy impact support.
Finally, procurement behavior is shifting toward outcomes and operational resilience. Instead of evaluating only match accuracy in controlled conditions, organizations are prioritizing deployment realities such as throughput at peak hours, failure-to-enroll rates across diverse populations, sensor durability, and uptime across distributed sites. This “total cost of operation” focus is reshaping vendor roadmaps toward serviceability, remote management, and integration readiness.
US tariff conditions in 2025 reshape device economics, sourcing strategies, and lifecycle planning for palm vein biometric hardware and components
United States tariff dynamics in 2025 are likely to influence palm vein biometric deployments primarily through the cost and availability of upstream components rather than through the matching algorithms themselves. Many palm vein systems depend on specialized near-infrared emitters, imaging sensors, microcontrollers, and secure elements that are sourced through global electronics supply chains. When tariffs affect particular categories of imported electronics, organizations can experience price adjustments, longer lead times, or forced redesigns to maintain margin and compliance.In response, buyers are becoming more attentive to bills of materials, country-of-origin disclosures, and supplier diversification. Programs that previously standardized on a single device model may now require dual sourcing or alternate form factors to reduce procurement risk. This can have downstream implications for integration, as different device families may require distinct SDK versions, drivers, or enclosure and mounting standards.
Tariff-related uncertainty also elevates the importance of lifecycle planning. If replacement parts and identical units become harder to procure, organizations may face challenges maintaining homogeneous deployments across sites, which can complicate user experience and operations. Consequently, procurement teams are placing greater emphasis on forward-compatible firmware policies, device management tooling, and long-term availability commitments.
On the vendor side, tariff pressure can accelerate nearshoring or final-assembly shifts, and it may incentivize more modular designs that allow substitution of impacted components. Over time, this could encourage a more resilient ecosystem, but in the near term it may create uneven pricing and delivery performance across suppliers. Leaders who anticipate these dynamics can reduce disruption by structuring contracts with flexibility, validating alternates early, and aligning deployment schedules with supply chain realities.
Segmentation reveals adoption is driven by modality choice, authentication workflow, deployment architecture, and end-use context rather than accuracy claims alone
Segmentation highlights that palm vein biometrics is not a single uniform market but a set of deployment patterns shaped by device architecture, operating environment, and the identity journey stage being secured. Across hardware, software, and services, buyers increasingly treat hardware as the capture and trust anchor, software as the matching and policy layer, and services as the glue that determines implementation success, especially when integrating with existing access control and identity platforms.From a technology perspective, contactless palm vein recognition is gaining priority where hygiene perception, throughput, and user acceptance are central, while contact-based implementations continue to appear in tightly controlled environments that value fixed hand positioning. Authentication modes such as one-to-one verification and one-to-many identification drive different infrastructure and governance needs; verification aligns well with badge-backed workflows and step-up authentication, while identification raises stronger privacy and watchlist governance questions that require clear purpose definition.
End-use segmentation shows distinct value propositions. In banking and financial services, palm vein supports strong customer authentication at branches and can reduce account takeover risk when combined with transaction risk engines. In healthcare, it can streamline patient identification and reduce duplicate records, but it must be deployed with rigorous consent and privacy safeguards. Government and law enforcement use cases emphasize high assurance and auditability, while corporate and industrial settings prioritize reliable access control, time and attendance integrity, and operational continuity in harsh environments.
Deployment models also differentiate adoption. On-premises implementations remain prevalent where organizations want direct control over biometric templates and matching infrastructure, whereas cloud-supported architectures are expanding as vendors provide configurable data residency options, encryption, and tenant isolation. Integration pathways with identity and access management, physical access control systems, and workforce management platforms often determine time-to-value more than sensor specifications. As a result, procurement increasingly evaluates SDK maturity, documentation quality, reference integrations, and the vendor’s ability to support upgrades without breaking existing workflows.
User experience segmentation adds another layer: fixed terminals at entrances, embedded modules within kiosks, and mobile-adjacent capture designs each introduce different ergonomics and failure modes. Leaders who map these segmentation factors to their operational constraints tend to achieve better enrollment quality, higher match performance in real settings, and fewer exceptions that force manual override processes.
Regional adoption differs by privacy expectations, infrastructure readiness, and integration ecosystems across the Americas, EMEA, and Asia-Pacific
Regional dynamics show that palm vein biometrics adoption is shaped by different combinations of regulation, infrastructure maturity, and cultural acceptance of biometrics. In the Americas, enterprise security modernization and financial services authentication are important catalysts, while privacy and biometric information laws elevate the need for strong governance, vendor transparency, and well-defined retention practices. Buyers often prioritize solutions that integrate cleanly with existing identity platforms and physical security infrastructure across distributed facilities.Across Europe, the Middle East, and Africa, the balance between security ambitions and privacy compliance strongly influences deployment design. Many organizations emphasize purpose limitation, clear consent flows, and data minimization, which favors architectures that reduce centralized exposure of biometric templates and provide auditable controls. In parts of the Middle East, large-scale infrastructure and smart facility initiatives can support higher-volume deployments, while in other areas, adoption is moderated by procurement cycles and integration complexity.
In Asia-Pacific, strong interest in frictionless user experiences, high-density transit and campus environments, and technology-forward service models can accelerate uptake. This region often sees experimentation with embedded and kiosk-based deployments, particularly in customer-facing contexts where speed and convenience matter. At the same time, organizations remain attentive to localization needs, including language support, regional certifications, and compatibility with local identity ecosystems.
Taken together, these regional patterns reinforce a central lesson: successful scaling depends on aligning the operating model with local expectations for privacy, security, and service delivery. Vendors and buyers that treat regional requirements as first-class design inputs-not afterthoughts-tend to avoid rework and reduce approval delays.
Leading vendors compete through secure edge hardware, integration-ready matching platforms, and partnerships that turn pilots into durable deployments
Company strategies in palm vein biometrics increasingly cluster around three themes: sensor and device innovation, platform-centric software differentiation, and ecosystem partnerships. Hardware-focused players compete on imaging quality, robustness in varied lighting and environmental conditions, and ergonomics that reduce user error during capture. Many also invest in anti-spoofing measures, secure enclaves, and tamper resistance to strengthen trust at the edge.Software and platform providers differentiate through matching performance under real-world constraints, scalable template management, and integration layers that connect to IAM, PACS, and workforce systems. Buyers are placing higher value on configurable policy engines, detailed audit logs, and role-based administration that supports both IT security and physical security teams. Increasingly, vendors position palm vein as one component in multimodal authentication suites, enabling organizations to tailor assurance levels without forcing a single modality across all users.
Partnership ecosystems are becoming decisive. Companies that build certified integrations with access control vendors, identity providers, kiosk manufacturers, and system integrators can reduce deployment friction and improve uptime through shared support models. In parallel, professional services capabilities-enrollment program design, site surveys, operator training, and privacy documentation support-are often the practical difference between a successful rollout and a stalled pilot.
Competitive intensity is also rising around compliance readiness and transparency. Vendors that provide clear documentation on template storage, encryption, key management, and data lifecycle controls are better positioned for procurement scrutiny. As more organizations formalize biometric governance, providers with mature tooling for consent management, data subject requests, and incident response alignment can convert trust into long-term contracts.
Leaders can de-risk adoption through risk-based use-case selection, resilient procurement, disciplined enrollment operations, and privacy-first governance
Industry leaders can strengthen outcomes by starting with a risk-based use-case hierarchy rather than defaulting to enterprise-wide rollout. High-impact entry points typically include controlled access points, high-value transactions, or identity recovery workflows where improved assurance directly reduces fraud and operational loss. From there, organizations can expand only after validating enrollment quality, exception handling, and throughput under peak conditions.Procurement should be structured to reduce supply chain and lifecycle risk. Contracts that address component substitution policies, long-term device availability, firmware update commitments, and security patch timelines help prevent fragmentation across sites. In parallel, teams should require evidence of secure template handling, including encryption in transit and at rest, key management practices, and clear separation of duties for administrators.
Operational design deserves equal attention. Enrollment is a major determinant of long-term matching performance, so leaders should invest in standardized enrollment scripts, staff training, and quality checks that catch poor captures early. Exception paths must be designed for continuity without undermining security, with clear policies for fallback authentication, supervisor overrides, and audit trails.
Finally, governance must be proactive. Privacy impact assessments, consent language, data retention schedules, and incident response playbooks should be established before scaling. Organizations that align legal, security, HR, and facilities stakeholders early can avoid later resistance and ensure the deployment remains compliant as regulations and internal policies evolve.
A structured methodology blends technical validation, ecosystem mapping, and governance assessment to produce decision-ready palm vein market insights
The research methodology for this executive summary’s themes follows a structured approach that triangulates technology realities, buyer priorities, and vendor capabilities. It begins with a clear definition of palm vein biometrics scope, including device types, matching software, integration layers, and supporting services across both physical and digital identity contexts. That scoping step is essential to avoid conflating palm vein solutions with unrelated biometric modalities or generic access control systems.Next, the analysis draws on systematic collection of publicly available technical documentation, regulatory and standards materials, and vendor disclosures related to security controls, deployment models, and interoperability. This is complemented by a structured review of product positioning, partner ecosystems, certification statements, and implementation patterns to understand how solutions are operationalized in the field.
To translate information into decision-useful insights, findings are normalized into comparable dimensions such as deployment complexity, integration readiness, governance maturity, and operational resilience. Cross-checks are performed to ensure claims are consistent with known constraints of near-infrared imaging, template management, and real-world capture ergonomics. The methodology emphasizes current practices, security expectations, and adoption drivers while avoiding unsupported assumptions.
Finally, insights are synthesized into narrative guidance designed for executives and technical stakeholders. The result is a practical lens on how palm vein biometrics is deployed, what is changing in the ecosystem, and where risks and opportunities typically concentrate during evaluation and scale-up.
Palm vein biometrics becomes a durable identity assurance layer when aligned to real-world operations, secure governance, and resilient deployment planning
Palm vein biometrics is increasingly positioned as a high-assurance modality that can reduce fraud and strengthen access control without imposing heavy user friction. Its internal-pattern capture provides inherent advantages in spoof resistance and reliability, particularly when deployments are engineered with strong enrollment practices and secure template governance.At the same time, the landscape is evolving quickly. Edge processing, multimodal strategies, and tighter privacy requirements are shaping product roadmaps and procurement criteria. In parallel, tariff and supply chain pressures in 2025 reinforce the need for sourcing resilience and lifecycle planning, especially for hardware-dependent deployments.
Organizations that succeed treat palm vein not as a standalone gadget but as an identity system component with clear policies, robust integrations, and measurable operational outcomes. When aligned to the right use cases and supported by rigorous governance, palm vein biometrics can become a durable part of modern identity assurance strategies across both physical and digital environments.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Palm Vein Biometrics Market
Companies Mentioned
The key companies profiled in this Palm Vein Biometrics market report include:- 4G Identity Solutions
- Alcatraz AI
- BioEnable Technologies Pvt. Ltd.
- BioSec Group Ltd
- Dakar Software Systems
- ePortation, Inc.
- Fujitsu Limited
- Hitachi, Ltd.
- IdentyTech Solutions Ltd.
- iDLink Systems Pte Ltd
- M2SYS Technology
- Mantra Softech (India) Private Limited
- Matrix COSEC
- Mofiria Corporation
- NEC Corporation
- Precise Biometrics AB
- Recogtech B.V
- Siemens AG
- Thales Group
- ZKTECO CO., LTD
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
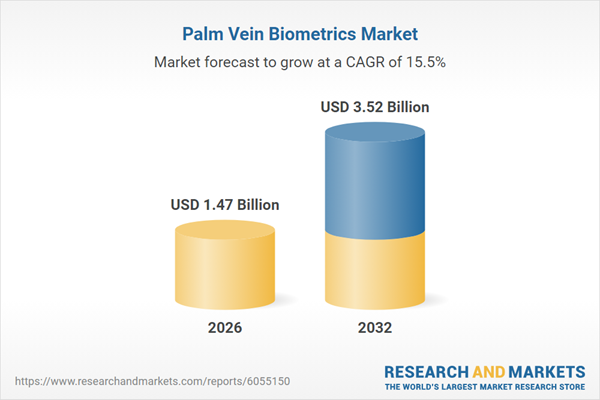
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 1.47 Billion |
| Forecasted Market Value ( USD | $ 3.52 Billion |
| Compound Annual Growth Rate | 15.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |