Speak directly to the analyst to clarify any post sales queries you may have.
The petri dish automation system market is rapidly advancing, driven by laboratories’ increasing need for efficiency, precision, and scalable operations. Adopting automated petri dish systems is transforming microbiology workflows by reducing manual intervention and minimizing operational variability, while supporting regulatory compliance and process traceability.
Market Snapshot: Petri Dish Automation System Market
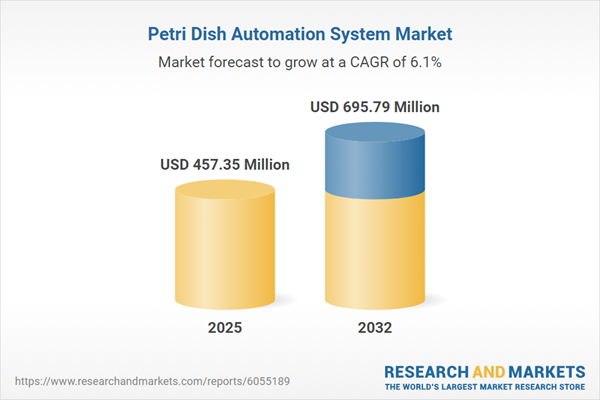
The global petri dish automation system market grew from USD 432.36 million in 2024 to USD 457.35 million in 2025. It is forecast to expand at a CAGR of 6.12%, reaching USD 695.79 million by 2032. This robust growth highlights increasing demand across biotechnology, pharmaceutical, and research sectors as organizations seek seamless, reliable automation to accelerate high-volume sample processing.
Scope & Segmentation
This report offers detailed insights into developments shaping the automated petri dish system landscape. Key areas covered include product innovations, technology adoption, end-user applications, and expansion across global regions. The primary keyword, “petri dish automation system market,” and the secondary keywords are addressed throughout.
- Component: Hardware (cleaning and sterilization systems; dispensing systems; incubation systems; labeling and tracking systems; plate handling systems)
- Software: Analytical tools, data management, and user interface design
- Technology: Optical imaging; robotic systems (arm-based and module-based robotic systems)
- Type of Automation: Fully automated and semi-automated systems
- End User: Biotechnology companies (manufacturing, quality control, research and development); pharmaceutical companies (clinical trials, drug discovery, quality assurance); research laboratories (academic and private research)
- Regional Coverage: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru); Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya); Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Leading Companies: Agilent Technologies, Beckman Coulter, bioMérieux, Formulatrix, Hamilton Company, Kawasaki Heavy Industries, SARSTEDT, Tecan Group, and others
Key Takeaways
- Automated petri dish systems integrate advanced robotics with intelligent software, optimally supporting high-throughput laboratory operations and consistent results.
- Configurable system architectures help laboratories adapt swiftly to evolving research priorities and project scales, without substantial capital investment in new equipment.
- AI-driven imaging and cloud-based interfaces are empowering remote monitoring, process optimization, and real-time quality assurance for distributed teams.
- Sustainability initiatives are reshaping equipment choices as energy-efficient components and lower-waste consumables align with corporate environmental goals.
- Strategic acquisitions and technology partnerships facilitate faster innovation cycles, helping both established leaders and agile newcomers meet complex end-user needs.
- Supplier diversification and collaborative procurement strategies are becoming essential as organizations respond to shifting trade policies and strive for supply chain resilience.
Tariff Impact: Navigating Regulatory Change
Recent tariff implementations in the United States have influenced costs for imported components such as optical imaging modules and robotic actuators. Some manufacturers are responding by increasing domestic assembly and establishing local production partnerships to control costs and maintain supply reliability. These dynamics are prompting more flexible sourcing and risk-mitigation strategies throughout the industry.
Methodology & Data Sources
This market research adopts a multi-stage methodology combining secondary research, in-depth interviews with laboratory automation specialists, and expert panel reviews. Data were triangulated from industry literature, corporate reporting, and regulatory publications to ensure reliable, actionable intelligence.
Why This Report Matters
- Supports senior leaders in identifying strategic investment opportunities within the evolving automated petri dish system ecosystem.
- Informs operational decisions by benchmarking technology adoption and market penetration across regions and end-user segments.
- Enhances supplier and procurement strategies by clarifying the implications of regulatory changes and innovation trends.
Conclusion
With laboratory demands evolving, the adoption of automated petri dish systems offers a pathway to operational excellence and future-proof scientific capabilities. Organizations that strategically invest in innovation and adaptability will position themselves for sustained competitive advantage.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Petri Dish Automation System market report include:- Agilent Technologies, Inc.
- b + b Automation and Control Technology GmbH
- BBS Automation GmbH by Dürr Group
- Beckman Coulter, Inc. by Danaher Corporation
- bioMérieux SA
- Don Whitley Scientific Limited.
- Formulatrix, Inc.
- Hamilton Company
- Hudson Robotics, Inc. by Argosy Healthcare Partners
- JEL CORPORATION
- Kawasaki Heavy Industries, Ltd.
- Microtechnix International, Inc.
- ONLINE Engineering inc.
- SANTSAi
- SARSTEDT AG & Co. KG
- SciRobotics Ltd.
- SHASHIN KAGAKU CO.,LTD.
- Tecan Group
- TMA AUTOMATION Sp. z o.o.
- Ybo Technologies Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 457.35 Million |
| Forecasted Market Value ( USD | $ 695.79 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |