Speak directly to the analyst to clarify any post sales queries you may have.
The antimicrobial peptides market is undergoing a significant transformation, propelled by innovations in biotechnology and increased urgency for addressing multidrug resistance. This evolving sector draws attention from stakeholders across pharmaceuticals, agriculture, food, and cosmetics, as organizations seek advanced solutions to emerging global health threats.
Market Snapshot: Antimicrobial Peptides Market Growth and Dynamics
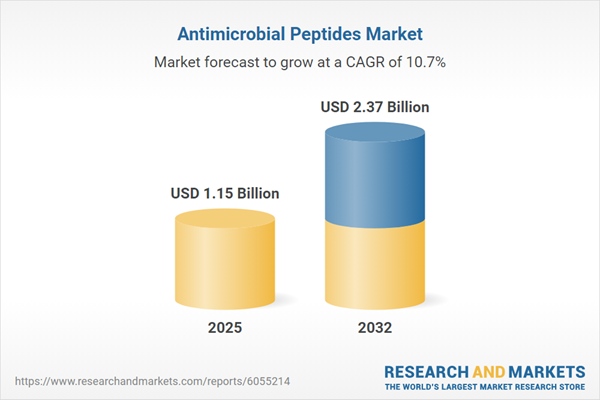
The antimicrobial peptides market grew from USD 1.05 billion in 2024 to USD 1.15 billion in 2025 and is expected to maintain a robust CAGR of 10.71%, reaching a projected value of USD 2.37 billion by 2032. This momentum reflects expanded adoption across sectors, increased R&D investments, and adaptability to evolving therapeutic challenges.
Scope & Segmentation of the Antimicrobial Peptides Market
This report provides comprehensive coverage of the antimicrobial peptides ecosystem, with multifaceted segmentation to aid strategic planning:
- Source: Peptides derived from animals, microbial secretions, plant extracts, and synthetic engineering.
- Structure: Includes alpha-helical, beta-sheet, and looped designs addressing functional diversity and therapeutic application.
- Mechanism of Action: Encompasses intracellular targeting and membrane pore formation, offering distinct approaches to microbial inhibition.
- Application: Covers agriculture (biopesticides), cosmetics (preservatives), food (natural antimicrobial agents), and pharmaceuticals (antibacterial, antifungal, antiviral, anticancer, immunomodulation, wound healing).
- Region: Evaluates the Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), and Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Leading Companies: Examines trends and activities among key players such as AbbVie Inc., AstraZeneca plc, Bayer AG, Boehringer Ingelheim International GmbH, Eli Lilly and Company, Johnson & Johnson Services, Inc., Novartis AG, Pfizer Inc., and others.
Key Takeaways for Senior Decision-Makers
- Antimicrobial peptides present advanced therapeutic mechanisms, including membrane disruption and intracellular inhibition, that expand drug developers' arsenal against resistant pathogens.
- Innovation in peptide design, such as solid-phase and recombinant synthesis, has accelerated scalability and minimized production costs, driving commercial viability across multiple industries.
- Emerging collaborations link biotechnology firms, academia, and contract development organizations, creating synergies for pipeline acceleration and bridging gaps between early discovery and clinical advancement.
- Versatile applications extend the value of antimicrobial peptides beyond pharmaceuticals, enabling market growth in food preservation, agriculture, and cosmetics.
- Strategic regional differences influence adoption pathways, with North America and Asia-Pacific exhibiting leadership in research-translation and manufacturing, respectively, while EMEA partners focus on public health solutions and regulatory harmonization.
Tariff Impact: U.S. Trade Policy Shifts and Supply Chain Strategies
New trade tariffs introduced by the United States in 2025 have reshaped supply chain economics for antimicrobial peptide producers. Elevated costs for imported amino acid precursors and contract manufacturing services have prompted organizations to optimize sourcing strategies, explore reshoring solutions, and forge long-term agreements in tariff-neutral territories. These tariff-driven adjustments have also sparked renewed focus on local manufacturing and agile inventory practices, reducing exposure to future trade risks and enhancing overall supply chain resiliency.
Antimicrobial Peptides Market: Methodology & Data Sources
This analysis combines rigorous secondary research from regulatory publications, patent filings, scientific journals, and corporate disclosures with targeted primary interviews among industry leaders, researchers, and regulatory experts. Data triangulation validates findings and trend models, while scenario analyses address critical operational and policy variables. Methodological robustness ensures accuracy and relevance for strategic decision-making.
Why This Report Matters for Senior Leadership
- Informs cross-sector strategy by analyzing emerging trends, competitive activities, and breakthrough technologies in the antimicrobial peptides market.
- Supports market-entry and expansion decisions through detailed segmentation, highlighting untapped opportunities and risk factors by source, structure, application, and region.
Conclusion
The antimicrobial peptides market is evolving rapidly in response to new therapeutic demands, technological innovation, and global health priorities. Strategic adaptation and partnership will define leadership and unlock new value across industries poised for sustainable growth.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Antimicrobial Peptides market report include:- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amferia AB
- AnaSpec Inc.
- AstraZeneca plc
- Bayer AG
- Boehringer Ingelheim International GmbH
- Celdara Medical, LLC
- Eli Lilly and Company
- EnBiotix Inc.
- Hello Bio
- Ingenza Limited
- Johnson & Johnson Services, Inc.
- Magainin Pharmaceuticals, Inc.
- Matrubials Inc.
- MaxWell Biosciences
- Meddenovo Drug Design
- Melinta Therapeutics
- Merck KGaA
- Novartis AG
- Novo Nordisk A/S
- Nuritas Ltd.
- Peptilogics
- Pfizer Inc.
- Toagosei Co Ltd
- Vertex Pharmaceuticals Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.15 Billion |
| Forecasted Market Value ( USD | $ 2.37 Billion |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |