Speak directly to the analyst to clarify any post sales queries you may have.
Embarking on a Comprehensive Introduction to Polyvinyl Siloxane Impression Material Market Fundamentals Benefits and Strategic Scope
Polyvinyl siloxane impression materials are elastomeric compounds widely utilized in dentistry for capturing precise replicas of oral tissues. Their viscoelastic properties, combined with excellent tear strength and dimensional stability, have established them as a gold standard for restorative, prosthetic, and orthodontic procedures. The silicone base renders them inert in the oral environment, minimizing allergic reactions and ensuring patient comfort. Moreover, their detailed reproduction of fine anatomical features supports predictable downstream processes, reducing corrective adjustments and chair time.This introduction outlines the fundamental makeup and clinical relevance of these materials, tracing their evolution from early silicone formulations to modern iterations that incorporate custom additives and advanced polymer matrices. Dental professionals have embraced their reproducibility, with fast-setting and regular-setting variants addressing diverse procedural needs. As laboratories and clinics seek ever greater fidelity, polyvinyl siloxane formulations continue to advance through iterative R&D efforts, yielding improvements in working times and hydrophilicity. These developments align with broader objectives of operational efficiency and patient satisfaction.
Moving forward, readers will gain strategic perspective on the market context and underlying drivers that define adoption dynamics and competitive positioning. By synthesizing recent innovations and regulatory considerations, this overview establishes a foundation for understanding subsequent transformations, tariff implications, segmentation intricacies, regional differentiators, and actionable insights that support informed decision making across the value chain.
In parallel, the convergence with digital dentistry technologies-such as intraoral scanners and 3D printing-has expanded the role of polyvinyl siloxane materials as benchmarks for calibration and validation of digital workflows. Their predictable rheological characteristics serve as reference standards in hybrid analog-digital processes, reinforcing their indispensability in contemporary practice.
Navigating Transformative Shifts Reshaping the Polyvinyl Siloxane Impression Material Landscape from Technological Breakthroughs to Operational Adaptations
Recent years have witnessed transformative innovations in polyvinyl siloxane formulations, as material scientists refine polymer crosslinking and incorporate novel surfactants to enhance hydrophilicity and tear resistance. Advanced catalysts now enable more precise setting times, while bioinspired additives improve marginal detail capture under moist conditions. These breakthroughs support seamlessly integrated workflows, bridging conventional impression techniques with burgeoning digital dentistry tools such as intraoral scanners and additive manufacturing systems.Alongside chemistry-driven advances, manufacturers have embraced operational agility to navigate evolving supply chains and cost structures. Strategies include diversifying raw material sources, implementing just-in-time inventory management, and deploying predictive analytics to anticipate demand fluctuations. Sustainable production practices-ranging from solvent reduction to incorporation of recyclable packaging-reflect industry commitments to environmental stewardship without sacrificing performance or regulatory compliance. Such shifts enhance reliability for clinical users and reinforce brand reputations in key markets.
Regulatory landscapes have also evolved, with harmonized standards emerging across major jurisdictions. Updated quality management protocols and revised biocompatibility criteria demand rigorous documentation and validation, prompting cross-functional collaboration between R&D teams and regulatory affairs specialists. This alignment streamlines product registrations and accelerates time to market for next-generation silicones, while reinforcing end-user confidence in safety and efficacy.
Together, these interconnected transformations establish a dynamic environment in which technological, operational, and regulatory forces coalesce. Understanding these shifts is essential for stakeholders seeking to navigate competitive pressures, optimize investment decisions, and align strategic priorities with evolving market realities.
Assessing the Cumulative Impacts of Upcoming United States Tariffs on the Polyvinyl Siloxane Impression Material Ecosystem in the Year Ahead
In response to changing trade policy frameworks, the introduction of cumulative tariffs by the United States has exerted material pressure on the polyvinyl siloxane supply chain. Raw inputs such as high-purity vinyl compounds and proprietary catalyst systems are subject to elevated import duties, elevating production costs for dispersions and base polymers. This imposition has prompted manufacturers to reassess supplier agreements and anticipate adjustments to factory gate pricing.Supply chain disruptions have been compounded by logistical complexities, as shippers realign to avoid tariff triggers and domestic producers seek to fill gaps. Some material formulators have accelerated qualification of alternative feedstock sources, including regional facilities in North America, to safeguard continuity. These strategic sourcing initiatives often entail upfront investments in validation and certification, but they yield longer-term resilience against future trade uncertainties. Concurrently, distributors and contract laboratories are adapting inventory strategies to mitigate exposure to price volatility.
As production costs rise, manufacturers face decisions regarding cost absorption versus downstream price adjustments. While some incumbent players have elected to consolidate production efficiencies to preserve margin, others have partially passed on incremental expenses to dental clinics and laboratories. This approach has elicited varied responses from end users, influencing procurement cycles and prompting engagement around volume rebates or extended payment terms to alleviate cash flow constraints.
Looking ahead, stakeholders will need to reconcile short-term fiscal impacts with broader market objectives. By exploring collaborative partnerships, investing in process innovations, and engaging in dialogue with policy advisors, decision-makers can position their operations to withstand tariff pressures while pursuing sustained growth and operational excellence.
Extracting Deep Segmentation Insights to Illuminate How Product Types Setting Speeds Sales Channels and End User Applications Drive Market Dynamics
A nuanced exploration of product type reveals distinct value propositions for heavy body, light body, and medium body formulations. Heavy body variants continue to serve as the workhorse for tray-based impressions due to their robustness and controlled flow, ensuring stability in multi-step workflows. In contrast, light body compounds are gaining momentum for margin detail reproduction in crown and bridge procedures, driven by enhancements in viscosity control. Medium body intermediates find favor in two-step techniques, offering a compelling balance between flowability and dimensional integrity.Examining setting time dynamics, fast-setting configurations address the demand for streamlined chairside efficiency by reducing working and setting periods without compromising tear strength. These rapid cures are particularly appealing in high-throughput clinical settings, where patient comfort and reduced appointment duration are paramount. Regular-setting formulations retain their appeal for complex cases that require extended working windows, enabling practitioners to achieve meticulous placement before polymerization.
The sales channel dimension underscores the coexistence of established offline networks with emerging online procurement platforms. Traditional distributors maintain strong relationships with clinical and laboratory buyers who value personal service and technical support, whereas digital ordering portals offer streamlined procurement and competitive pricing for bulk buyers. This duality reflects a broader shift toward omnichannel strategies that leverage both face-to-face engagement and e-commerce efficiencies.
Finally, end-user applications span dental clinics, dental laboratories, and hospital environments, each with unique priorities. Clinics prioritize ease of use and turnaround times, laboratories emphasize reproducible cast accuracy, and hospitals focus on biocompatibility and compliance. Integrating these segmentation perspectives offers a comprehensive framework for tailoring product development, marketing outreach, and service models to specific stakeholder needs.
Unveiling Critical Regional Insights to Highlight Distinct Market Characteristics Across the Americas Europe Middle East Africa and Asia Pacific Jurisdictions
Within the Americas, robust dental infrastructure and established reimbursement frameworks underpin sustained demand for premium elastomeric materials. North American markets are marked by high penetration of digital workflows, with clinicians and laboratories leveraging intraoral scanning and CAD/CAM integration. This environment fosters continuous product innovation, as manufacturers tailor silicone formulations to complement digital model systems. Latin American markets, while more price sensitive, are exhibiting gradual uptake of mid-tier products as economies stabilize and private dental expenditure rises.The Europe, Middle East, and Africa region presents a diverse landscape characterized by regulatory convergence alongside pronounced market fragmentation. Western European countries adhere to stringent material standards and clinical guidelines, driving demand for premium grade silicones. In contrast, emerging Middle Eastern markets are propelled by significant infrastructure investments and growing medical tourism sectors. Select African markets, constrained by budgetary limitations, favor cost-effective solutions with essential performance attributes. Across the region, harmonized CE marking requirements facilitate cross-border distribution, albeit with localized compliance nuances.
In Asia-Pacific, rapid urbanization and expanding dental clinic networks have fueled market acceleration, particularly in metropolitan centers. Market participants navigate complex regulatory regimes that vary widely between nations, prompting strategic alliances with local distributors. Cost sensitivity remains a defining feature, encouraging adoption of tiered offerings that balance affordability with performance. Simultaneously, increasing patient awareness and rising per capita disposable income support demand for advanced impression materials, creating opportunities for premium product lines.
Anchoring these regional insights provides a strategic vantage point for aligning production, marketing, and investment decisions. By appreciating distinct market drivers across the Americas, Europe Middle East & Africa, and Asia-Pacific, stakeholders can optimize resource allocation and cultivate region-specific value propositions.
Revealing Key Company Strategies Innovations Partnerships and Competitive Positioning Shaping the Polyvinyl Siloxane Impression Material Marketplace
A cohort of multinational entities commands significant visibility through comprehensive portfolios that span heavy, light, and medium body variants, as well as fast-setting and regular-setting options. These corporations leverage extensive R&D capabilities to introduce next-generation silicone systems, often integrating proprietary catalyst formulations that enhance tear resistance and hydrophilicity. Their global production networks and established distribution channels enable them to maintain consistent supply while responding swiftly to regulatory updates and customer feedback.Concurrently, a number of mid-sized specialists have carved out niches by focusing on targeted end-user applications, such as high-precision laboratory workflows or hospital-based prosthetic procedures. These agile participants often capitalize on rapid innovation cycles and close collaboration with clinical thought leaders, enabling customized solutions that address specific procedural requirements. Their willingness to invest in localized production or co-development agreements further differentiates them in select geographies.
Strategic alliances and mergers continue to reshape the competitive landscape, as leading players seek to expand regional footprints and augment technical expertise. Joint ventures with regional distributors facilitate market entry in areas with stringent regulatory regimes, while acquisitions of niche formulators broaden product mixes and enhance service offerings. Such transactions reflect an overarching drive to consolidate supply chains and unify branding strategies across multiple jurisdictions.
Maintaining a competitive edge increasingly revolves around the ability to deliver end-to-end support, encompassing technical training, digital integration guidance, and post-sale troubleshooting. As differentiation shifts from purely product-focused to solution-oriented value propositions, companies that excel in knowledge transfer and customer engagement are poised to outperform. These dynamics underscore the blend of innovation, partnership, and service excellence that defines leadership in the polyvinyl siloxane impression material market.
Actionable Recommendations for Industry Leaders to Capitalize on Emerging Trends Operational Efficiencies and Strategic Growth Opportunities in the PVS Sphere
To capitalize on emerging market dynamics, industry leaders should prioritize sustained investment in research and development, concentrating on next-generation polymer chemistries that enhance accuracy, biocompatibility, and environmental sustainability. Collaborative initiatives with material science institutions and clinical research centers can accelerate product validation and shorten time to market. Simultaneously, integrating digital workflow compatibility into new formulations ensures seamless adoption alongside intraoral scanners and additive manufacturing technologies.Supply chain diversification represents another critical vector for resilience and cost containment. Organizations should evaluate regional sourcing alternatives for key vinyl precursors and catalyst systems, while building strategic reserves to buffer against tariff fluctuations and logistical bottlenecks. Concurrently, embracing sustainable manufacturing practices-such as solvent-free processing and recyclable packaging-can reduce environmental footprints and align brand reputations with evolving customer expectations.
Proactive regulatory engagement is essential for anticipating changes in global standards and expediting product approvals. Establishing dedicated cross-functional task forces that include regulatory affairs, quality assurance, and clinical affairs professionals enhances compliance readiness. Early dialogue with regulatory bodies and participation in standard-setting committees support smoother pathway navigation and reinforce credibility with end users.
Finally, strengthening end-to-end customer relationships through tailored training programs, real-time technical support, and collaborative marketing initiatives deepens market penetration. Digital platforms that facilitate knowledge sharing and case study dissemination empower practitioners to fully leverage product capabilities, fostering loyalty and advocacy. By balancing product innovation with operational excellence and stakeholder engagement, industry leaders can navigate complexities and unlock sustainable growth trajectories.
Detailing Robust Research Methodology Data Collection Techniques Analysis Frameworks and Validation Processes Underpinning the PVS Impression Material Study
The study employs a robust mixed-methods approach to deliver a comprehensive analysis of the polyvinyl siloxane impression material landscape. Primary research underpins the investigation, encompassing in-depth interviews with key opinion leaders, procurement managers, and materials scientists across clinical, laboratory, and manufacturing settings. Structured surveys supplement these qualitative insights, enabling quantification of adoption drivers, purchasing criteria, and performance expectations across diverse end-user segments.Secondary research complements primary inputs through systematic examination of technical journals, regulatory filings, patent databases, and corporate publications. This review informs a granular understanding of historical product development trajectories, emerging formulation trends, and evolving compliance requirements. Additionally, public domain sources such as industry association reports and trade press articles provide context on macroeconomic factors and supply chain dynamics that influence market behavior.
Analytical rigor is maintained through rigorous data triangulation, where insights from primary and secondary channels are cross-referenced to validate findings and resolve discrepancies. Quantitative data points are synthesized using statistical frameworks, while qualitative perspectives are mapped through thematic analysis to capture nuanced stakeholder sentiments. Custom models assess segmentation attributes, regional performance nuances, and competitive intensity without relying on extrapolated market forecasting algorithms.
To ensure integrity and relevance, an expert advisory panel comprising dental practitioners, laboratory directors, and materials engineers reviews the methodology and interprets key observations. Iterative feedback loops facilitate refinement of assumptions and methodologies, culminating in a validated data foundation that supports strategic decision-making and actionable recommendations.
Drawing Together Key Findings Strategic Implications and Broader Perspectives in Support of Informed Decision Making in PVS Impression Applications
The analysis underscores how material science advances, operational agility, and regulatory evolution converge to redefine the polyvinyl siloxane impression material domain. From customized rheological profiles to integrated digital workflows, innovations in polymer formulation are enhancing accuracy and efficiency for clinicians and laboratory technicians alike. Concurrently, strategic supply chain realignments and sustainability imperatives are reshaping procurement and manufacturing paradigms.Regional and segmentation insights reveal that differentiated strategies are essential for navigating diverse market conditions. Stakeholders operating in the Americas must balance premium product offerings with cost containment, whereas participants in Europe, the Middle East, and Africa should tailor regulatory and distribution approaches to local nuances. In Asia-Pacific, growth opportunities hinge on cost-competitive yet high-performance solutions that resonate with expanding clinic networks and rising patient expectations.
Ultimately, the synthesis of key findings and actionable recommendations equips decision-makers with clarity on how to leverage technological breakthroughs, mitigate trade headwinds, and forge resilient partnerships. By adopting a forward-looking perspective and aligning operational capabilities with emerging market demands, entities across the value chain can position themselves to capture long-term value and drive sustained progress in the polyvinyl siloxane impression material sector.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Heavy Body
- Light Body
- Medium Body
- Setting Time
- Fast-setting PVS
- Regular-setting PVS
- Sales Channel
- Offline
- Online
- End-User Applications
- Dental Clinics
- Dental Laboratories
- Hospitals
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3M Company
- Crown Delta Corporation
- GC International AG
- Henry Schein, Inc.
- Hiossen Implant
- Kerr Corporation by Envista Holdings Corporation
- Kettenbach SNC
- Keystone Dental Group
- Pyrax Polymars
- Scott's Dental Supply
- Ultradent Products
- Zhermack GmbH by Dentsply Sirona Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Polyvinyl Siloxane Impression Material market report include:- 3M Company
- Crown Delta Corporation
- GC International AG
- Henry Schein, Inc.
- Hiossen Implant
- Kerr Corporation by Envista Holdings Corporation
- Kettenbach SNC
- Keystone Dental Group
- Pyrax Polymars
- Scott's Dental Supply
- Ultradent Products
- Zhermack GmbH by Dentsply Sirona Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
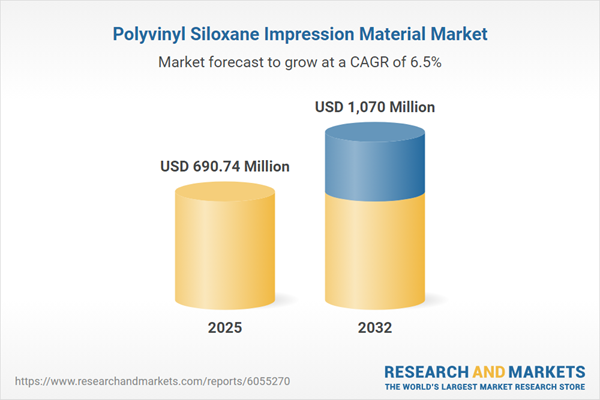
| Estimated Market Value ( USD | $ 690.74 Million |
| Forecasted Market Value ( USD | $ 1070 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |