Speak directly to the analyst to clarify any post sales queries you may have.
Tris buffer solutions have cemented their role as essential tools for maintaining pH stability across diverse scientific and industrial workflows. As demand for reliable buffering agents intensifies in advanced therapeutics and manufacturing, understanding market dynamics becomes crucial for informed, strategic decision-making.
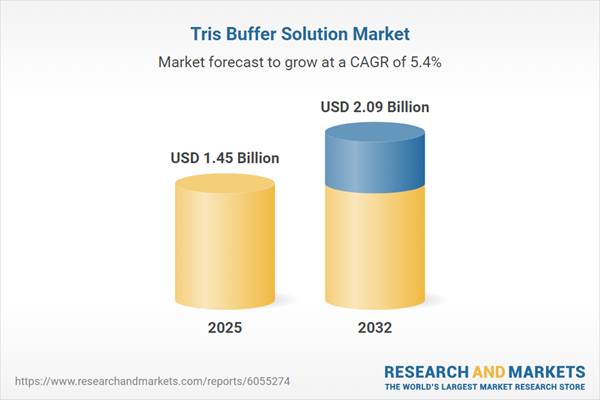
Market Snapshot: Tris Buffer Solution Market Growth Trajectory
The Tris Buffer Solution Market grew from USD 1.38 billion in 2024 to USD 1.45 billion in 2025. It is expected to continue growing at a CAGR of 5.36%, reaching USD 2.09 billion by 2032. Driving factors include escalating needs in molecular biology, pharmaceutical development, biotechnology, and precision manufacturing. Heightened end-user requirements for reagent quality, process reproducibility, and compliance have prompted increased investments and innovations across the buffer landscape.
Scope & Segmentation: In-Depth Market Structure
This report delivers comprehensive analysis across product derivatives, forms, purity, distribution, applications, end-users, and geographical regions.
- Derivative Types: Tris Acetate EDTA, Tris Borate EDTA, Tris Buffered Saline, Tris Glycine Buffer, Tris HCl, Tris-Glycine-Sodium Dodecyl Sulfate
- Form: Liquid, Powder, Tablet
- Purity Levels: High, Medium, Low
- Distribution Channels: Pharmacy Chains, Research Supply Wholesalers, Brand Websites, E-Commerce Platforms
- Applications: Cell Culture, Protein Purification, Chemical Processing, Laboratory Research, Pharmaceutical Production
- End Users: Biotechnology & Pharmaceutical Firms, Contract Research Organizations, Research & Academic Institutions
- Regions: North America (United States, Canada, Mexico), Latin America (Brazil, Argentina, Chile, Colombia, Peru), Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Key Companies: Agilent Technologies, Avantor, Bio-Rad Laboratories, Boster Biological Technology, Cayman Chemical, Dojindo Molecular Technologies, DUKSAN PHARMACEUTICAL INDUSTRIAL, FUJIFILM Wako Pure Chemical, GE Healthcare Technologies, Horizon Discovery, Junsei Chemical, Loba Chemie, Lonza Group, Merck KGaA, MP BIOMEDICALS, Nacalai Tesque, Promega Corporation, Qiagen, Santa Cruz Biotechnology, Sinopharm Group, Sisco Research Laboratories, SOLARBIO, Spectrum Chemical, Takara Bio, Thermo Fisher Scientific
Key Takeaways: Strategic Insights for Decision-Makers
- Tris buffer solutions are fundamental in sustaining pH consistency for sensitive molecular and cell-based research, supporting experimental repeatability and product quality.
- End-user demand for high-performance buffer grades is driving advancements in reagent formulation, quality management, and purity standards.
- Automation and miniaturized lab technologies have heightened the need for buffer compatibility with emerging instrumentation and high-throughput processes.
- Manufacturers are differentiating through sustainable production, customizable kits, and responsive supply strategies addressing both regulatory and environmental priorities.
- Comprehensive documentation, traceability, and regional regulatory adherence are increasingly vital, shaping supplier competitiveness in every major market.
Tariff Impact on Supply Chains and Cost Structures
Recent U.S. tariff adjustments on precursor chemicals have affected procurement and manufacturing costs throughout the tris buffer supply chain. These changes prompted producers to reevaluate sourcing models, adopt alternative supplier networks, and negotiate pricing agreements. As a result, supply chain diversification and collaborative logistics now play a larger role in preserving market stability and access for critical research and production activities.
Methodology & Data Sources
This report leverages primary research through interviews with R&D leaders and regulatory experts, combined with secondary analysis of journals, industry whitepapers, trade association insights, and regulatory filings. Analytical models, such as SWOT and Porter’s Five Forces, provide a multidimensional view of market intensity and supplier dynamics.
Why This Report Matters
- Enables executives to pinpoint growth areas, mitigate supply chain risks, and adapt strategies to evolving regulatory and technology trends.
- Supports purchasing, procurement, and innovation decisions with segmented, actionable intelligence and competitor benchmarking.
- Offers complete visibility into product differentiation, emerging markets, and compliance challenges relevant to senior stakeholders.
Conclusion
The Tris Buffer Solution Market is positioned for steady growth as applications and compliance requirements evolve globally. This report equips leaders with the clarity to navigate shifts, prioritize investments, and advance operational resilience across the value chain.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Tris Buffer Solution market report include:- Agilent Technologies, Inc.
- Avantor, Inc.
- Bio-Rad Laboratories, Inc.
- Boster Biological Technology CO LTD.
- Cayman Chemical Co Inc.
- Dojindo Molecular Technologies, Inc.
- DUKSAN PHARMACEUTICAL INDUSTRIAL CO.
- FUJIFILM Wako Pure Chemical Corporation
- GE Healthcare Technologies, Inc.
- Horizon Discovery Ltd.
- Junsei Chemical Co., Ltd.
- Loba Chemie Private Limited
- Lonza Group
- Merck KGaA
- MP BIOMEDICALS, INC.
- Nacalai Tesque, Inc.
- Promega Corporation
- Qiagen N.V.
- Santa Cruz Biotechnology, Inc.
- Sinopharm Group Co., Ltd.
- Sisco Research Laboratories Private Limited
- SOLARBIO LIMITED
- Spectrum Chemical Mfg. Corp.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.45 Billion |
| Forecasted Market Value ( USD | $ 2.09 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |