Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for Enhanced Livestock Welfare Through Innovative Pain Relief Solutions and Preventive Strategies Across Diverse Production Systems
Pain management in livestock has evolved into a foundational pillar of modern animal health, reflecting heightened attention to welfare, productivity, and regulatory compliance. This introduction explores how stakeholders leverage pain relief and preventive strategies to address acute discomfort from routine procedures as well as chronic conditions that can undermine animal well-being. As global demand for ethically produced animal proteins rises, producers recognize that untreated pain not only affects animal health but also carries implications for growth performance, disease resistance, and consumer perceptions. Consequently, investment in analgesic protocols and preventive measures offers a dual benefit: reinforcing stewardship commitments while optimizing economic returns.Furthermore, integrated approaches combining pain relief agents, preventative modalities, and management practices have gained traction. Veterinary professionals increasingly adopt multimodal regimens that tailor therapeutic interventions to individual animal needs, procedural contexts, and species-specific sensitivities. Collaboration among pharmaceutical manufacturers, research institutions, and end-users has accelerated the development of novel formulations designed for targeted delivery and rapid onset of action. By laying this conceptual groundwork, readers will gain a clear view of the forces shaping pain mitigation across diverse production paradigms and appreciate the nexus between innovation, regulation, and on-farm implementation.
Transitioning from foundational considerations, subsequent sections will examine pivotal shifts, policy influences, and market stratifications that define the current landscape of livestock pain relief and prevention.
Emerging Dynamics Reshaping Livestock Pain Management Practices and Driving Transformational Shifts in Therapeutic Approaches Across Regions
Over the past decade, a convergence of research breakthroughs, regulatory reforms, and shifting consumer expectations has recalibrated priorities in livestock pain management. Advancements in pharmacology have introduced novel classes of analgesics, enabling more effective control of both acute and chronic pain without compromising safety profiles. Simultaneously, regulatory authorities have tightened guidelines governing permissible compounds, residue testing, and withdrawal periods, prompting manufacturers to invest heavily in compliance frameworks and alternative platforms.In parallel, societal demands for transparency and higher welfare standards have compelled producers to adopt on-farm protocols that demonstrate tangible benefits to animal comfort. Trailblazing operations have begun integrating pain assessment tools and welfare scoring metrics, thereby providing objective data to validate analgesic protocols. This shift underscores a broader trend toward data-driven decision-making, as stakeholders harness digital health monitoring and precision delivery technologies.
Moreover, collaboration between veterinary practitioners and feedlot managers has facilitated the emergence of holistic care models, where nutritional support, housing enhancements, and therapeutic interventions coalesce to optimize recovery. As a result, the industry stands at an inflection point: one where cutting-edge science converges with pragmatic farm-level adaptations to redefine how pain relief and prevention are operationalized across the supply chain. These transformative dynamics set the stage for exploring trade policy impacts, segmentation insights, and regional nuances in subsequent discussions.
Assessing the Ripple Effects of 2025 United States Tariffs on the Accessibility and Affordability of Pain Management Solutions for Livestock Producers
The introduction of new tariff measures by the United States in 2025 has created a ripple effect that extends well beyond customs declarations. Heightened duties on select pharmaceutical inputs and finished products have prompted manufacturers to reassess their supply chain footprints, evaluating the feasibility of local sourcing, contract manufacturing, and nearshoring. In turn, distribution networks have become increasingly agile, as companies negotiate revised freight agreements and explore alternative logistical routes to minimize cost escalation.Producers and distributors have responded with adaptive strategies, including forward purchasing arrangements, regional consolidation of inventory, and enhanced collaboration with third-party logistics providers. Veterinary service providers, in particular, have adjusted pricing structures to reflect incremental import costs while preserving accessibility for end-users. Even though some product classes experienced sticker-shock at the point of import, a combination of volume discounts and strategic alliances helped maintain continuity of care in critical production windows.
In parallel, the tariff landscape accelerated investments in domestic research and development, driving interest in local innovation hubs and partnerships with academic institutions. This onshore momentum has the potential to yield future generations of analgesic agents and novel delivery systems, thereby reducing vulnerability to international policy fluctuations. The interplay between tariff pressures and strategic adaptation underscores the resilience of the industry, which continues to safeguard animal welfare and operational viability despite evolving trade constraints.
Unveiling Critical Segmentation Perspectives Highlighting Therapeutic Categories Species Preferences Administration Routes and Source-Related Challenges in Livestock Pain Management
A nuanced understanding of product segmentation offers critical insights into how therapeutic strategies align with animal needs and operational imperatives. On the basis of type, treatments are distinguished between solutions formulated for acute pain, such as postoperative discomfort or procedural interventions, and those designed to address chronic pain syndromes, including inflammatory disorders and degenerative joint conditions. Concurrently, dissecting the source of pain yields distinctions between disease-related discomfort, injury-induced trauma, procedural stressors, and post-surgical inflammation, each demanding specific analgesic profiles and dosing regimens.Beyond therapeutic classification, delivery routes further differentiate offerings. Oral formulations facilitate convenient administration via feed or water, whereas parenteral options encompass injectable intramuscular and intravenous formulations that enable rapid systemic distribution. Topical preparations provide localized relief for surface lesions or sensitive skin areas, enhancing compliance and minimizing systemic exposure. Such method-of-administration diversity underpins the capacity to tailor interventions to herd management workflows and veterinary protocols.
Lastly, species-specific considerations drive targeted innovation. Cattle producers emphasize robust formulations that withstand extensive field handling, while poultry systems seek rapid-action modalities compatible with mass treatment approaches. Sheep and goat operations often require low-volume dosing solutions suited to smaller body masses, and swine producers prioritize applications that integrate seamlessly into biosecure environments. By weaving these segmentation dimensions together, stakeholders can refine product portfolios to meet distinct clinical, logistical, and welfare criteria across the livestock spectrum.
Deciphering Regional Differentiators in Livestock Pain Relief Adoption Patterns Across the Americas Europe Middle East Africa and the Asia-Pacific
Regional factors exert a profound influence on the adoption and evolution of pain relief protocols across the livestock sector. In the Americas, established regulatory frameworks and advanced distribution networks facilitate early uptake of innovative analgesics and preventive regimens. Producers in North and South America leverage integrated data platforms to validate product efficacy, while collaborative research initiatives support the development of context-specific solutions for cattle, swine, and poultry operations.Across Europe, the Middle East, and Africa, regulatory harmonization efforts continue to shape product approvals and residue management practices. European producers, in particular, operate under stringent welfare mandates that mandate documented pain mitigation measures, driving a premium on validated clinical outcomes. Conversely, emerging economies in the Middle East and Africa navigate a complex tapestry of import dependencies, cold-chain challenges, and resource constraints, fostering opportunities for cost-effective formulations and decentralized manufacturing.
In the Asia-Pacific region, rapid expansion of intensive livestock systems underscores the need for scalable pain relief strategies that align with high-volume production models. Local research collaborations have accelerated the introduction of novel delivery technologies, such as long-acting injectables and feed-based analgesics, while government incentives promote investment in veterinary infrastructure. As regional dynamics converge, the differential pace of adoption highlights both challenges and opportunities for cross-border partnerships and knowledge transfer.
Profiling Leading Innovators and Established Players Shaping the Future of Pain Relief and Prevention in Animal Health Through Strategic Collaborations and Product Advancements
Leading companies in animal health are demonstrating their commitment to pain relief and prevention through strategic alliances, targeted acquisitions, and robust research portfolios. Major global players have expanded their pipelines by integrating advanced non-steroidal options with next-generation analgesics, leveraging their established distribution channels to accelerate market penetration. Partnerships with biotechnology firms have also birthed innovative formulations, such as sustained-release injectables and nanoparticle-based topical therapies.Simultaneously, agile mid-tier organizations are carving out niches through specialized offerings tailored to regional needs. Their focus on rapid field trials, local regulatory expertise, and responsive customer support enables them to compete effectively alongside legacy brands. Collaborative ventures between these mid-tier innovators and academic centers have yielded proprietary platforms that optimize bioavailability while reducing environmental residues.
Furthermore, a wave of start-ups is emerging with disruptive business models, harnessing precision delivery devices, digital diagnostics, and telehealth-enabled veterinary services. These newcomers collaborate closely with integrators and feedlot operators to validate efficacy in real-world conditions, thereby accelerating adoption curves. Collectively, this spectrum of companies-from established multinational corporations to nimble specialists-fuels a competitive environment that prioritizes both scientific rigor and commercial agility.
Implementing Proactive Strategies and Collaborative Initiatives to Elevate Pain Relief Standards and Optimize Preventive Care Outcomes in Livestock Operations
Industry leaders can capitalize on evolving opportunities by adopting a proactive, multi-stakeholder approach that bridges research, regulation, and end-user engagement. First, fostering open innovation networks with academic institutions and contract research organizations will accelerate the translation of promising analgesic candidates into commercially viable solutions. By designing collaborative proof-of-concept trials, companies can de-risk development pathways and secure early regulatory feedback.Second, aligning product development roadmaps with emerging welfare guidelines and certification standards will strengthen market positioning and build trust among consumers. Integrating payloads that demonstrate clear benefits-such as reduced inflammation markers or shortened recovery times-into On-Label documentation will further enhance credibility with veterinary professionals.
Third, investing in digital platforms that enable real-time pain assessment and treatment tracking can differentiate offerings and support premium service models. Such tools empower producers to quantify welfare improvements, optimize dosing schedules, and validate return-on-investment metrics. By embracing these recommendations, industry leaders will not only advance animal welfare but also reinforce commercial resilience amid dynamic trade and regulatory landscapes.
Outlining the Rigorous Methodological Framework Underpinning Comprehensive Livestock Pain Relief and Prevention Research Utilizing Multisource Data Integration Techniques
The research methodology underpinning this analysis integrates diverse data sources, rigorous validation processes, and expert consultations to ensure a holistic perspective on livestock pain relief and prevention. Initially, comprehensive secondary research was conducted across scientific journals, regulatory filings, and industry whitepapers to map the therapeutic landscape and identify key knowledge gaps. This phase provided context on analgesic classes, welfare frameworks, and delivery innovations.Subsequently, primary research involved structured interviews and workshops with veterinary experts, farm managers, and pharmaceutical executives. These engagements yielded qualitative insights on regional adoption dynamics, logistical constraints, and emerging clinical trends. Data triangulation techniques were employed to cross-verify findings, ensuring consistency between field observations and published evidence.
Quantitative analysis leveraged proprietary databases tracking product launches, formulation attributes, and patent filings. Advanced filtering algorithms categorized offerings by type, source, administration mode, and target species. Finally, iterative peer reviews by independent animal health specialists validated the accuracy and relevance of the conclusions. This robust framework underwrites the reliability of the strategic recommendations and regional assessments presented herein.
Summarizing Transformative Insights and Reinforcing the Strategic Imperative for Advanced Pain Management Solutions to Enhance Livestock Welfare and Productivity
In conclusion, the intersection of scientific innovation, regulatory evolution, and trade policy dynamics has created a pivotal moment for pain relief and prevention in livestock management. Stakeholders now possess a clearer view of how acute and chronic pain interventions, segmented by type, source, administration route, and species, can be optimized to enhance animal welfare and operational efficiency. Regional insights highlight distinct adoption pathways across the Americas, Europe, the Middle East, Africa, and Asia-Pacific, underscoring opportunities for tailored market entry and collaborative research.Key players are leveraging strategic partnerships and advanced formulations to address emerging needs, while progressive producers adopt data-driven protocols to validate treatment efficacy. The cumulative impact of 2025 tariff adjustments has spurred supply chain resiliency and domestic innovation initiatives, reinforcing the industry's capacity to adapt under shifting economic conditions.
As the competitive landscape continues to evolve, embracing the actionable recommendations outlined in this summary will prove essential. By fostering open innovation, aligning with welfare standards, and deploying digital assessment tools, decision-makers can secure a sustainable path forward. These conclusions serve as a strategic compass for those committed to elevating livestock welfare and maintaining a robust veterinary therapeutics ecosystem.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Type
- Acute Pain
- Chronic Pain
- Source
- Disease-Related Pain
- Injury-Related Pain
- Procedural Pain
- Surgical Pain
- Method of Administration
- Oral
- Parenteral
- Intramuscular
- Intravenous
- Topical
- Target Species
- Cattle
- Poultry
- Sheep & Goats
- Swine
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AdvaCare Pharma USA
- Bimeda Holdings Limited
- Boehringer Ingelheim International GmbH
- Cargill, Incorporated
- Ceva Santé Animale
- Covetrus, Inc.
- Dechra Pharmaceuticals Limited
- Elanco Animal Health Incorporated
- HUVEPHARMA EOOD
- IDEXX Laboratories, Inc
- Merck & Co., Inc.
- Neogen Corporation
- Norbrook Group
- Patterson Vet Supply, Inc.
- Phibro Animal Health Corporation
- Vetoquinol S.A.
- Vetpro Healthcare
- VIRBAC Animal Health India Private Ltd
- Zoetis Services LLC
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Pain Relief & Prevention in Livestock market report include:- AdvaCare Pharma USA
- Bimeda Holdings Limited
- Boehringer Ingelheim International GmbH
- Cargill, Incorporated
- Ceva Santé Animale
- Covetrus, Inc.
- Dechra Pharmaceuticals Limited
- Elanco Animal Health Incorporated
- HUVEPHARMA EOOD
- IDEXX Laboratories, Inc
- Merck & Co., Inc.
- Neogen Corporation
- Norbrook Group
- Patterson Vet Supply, Inc.
- Phibro Animal Health Corporation
- Vetoquinol S.A.
- Vetpro Healthcare
- VIRBAC Animal Health India Private Ltd
- Zoetis Services LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
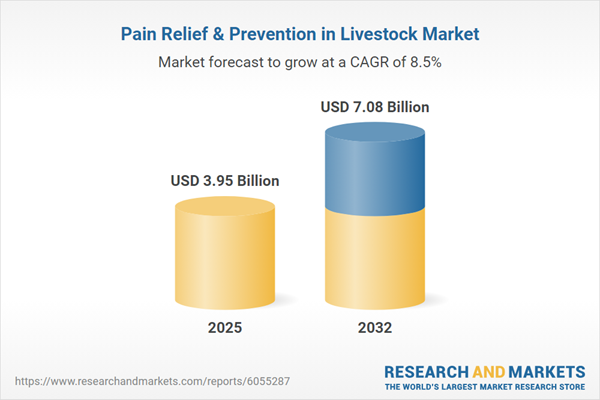
| Estimated Market Value ( USD | $ 3.95 Billion |
| Forecasted Market Value ( USD | $ 7.08 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |