Speak directly to the analyst to clarify any post sales queries you may have.
Medical smart rings are transforming digital health monitoring by delivering reliable, real-time insights for patient care and operational efficiency. Innovative, easy-to-adopt devices, they give senior decision-makers practical options for advancing healthcare services and optimizing resource management within organizations.
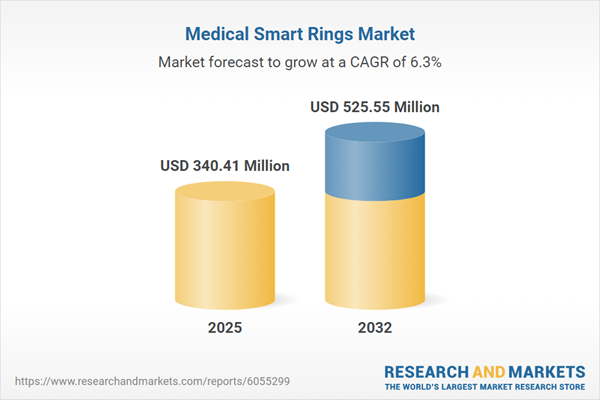
Market Snapshot: Medical Smart Rings Market Growth and Dynamics
The medical smart rings market is demonstrating steady expansion, currently valued at USD 321.45 million in 2024 and projected to reach USD 340.41 million in 2025. With a compound annual growth rate (CAGR) of 6.33%, the sector is forecasted to achieve a market size of USD 525.55 million by 2032. This upward movement is supported by increased demand for advanced health technologies, ongoing advancements in sensor integration, improvements in measurable clinical results, and the increasing influence of telehealth solutions. Active cross-border partnerships and investments are contributing to robust infrastructure improvements, paving the way for wider adoption and continual evolution in both clinical and wellness settings.
Scope & Segmentation of the Medical Smart Rings Market
This market research report provides actionable intelligence to support executive strategies on innovation, market positioning, and expansion. Comprehensive segmentation enhances precise analysis for diverse use cases and business goals:
- Product Types: Diagnostic smart rings enable detailed medical condition monitoring for clinical use; wellness-oriented rings empower individuals with tools for tracking chronic conditions; multifunctional rings offer bundled biometric monitoring and analytics capabilities in compact devices.
- Applications: Devices support clinical diagnostics for medical teams, personal fitness tracking for consumers, and proactive health management among wellness-focused users.
- End Users: Core segments comprise healthcare institutions seeking scalable remote assessments, consumers demanding instant biometric feedback, and sports professionals applying advanced analytics to optimize training.
- Regional Coverage: The market analysis spans the Americas, Europe-Middle East-Africa, and Asia-Pacific, supplying decision-makers with insight on key economies such as the United States, China, Germany, and Japan, particularly in relation to regulatory requirements and competitive landscapes.
- Technologies: Innovations include miniaturized biosensors for comfort, hybrid optical and electrical sensor arrays for precise data, energy harvesting systems to maximize battery life, machine learning algorithms for analytics, and platforms designed for seamless integration with electronic health records and telemedicine infrastructure.
Key Takeaways for Senior Decision-Makers
- Compact and precise sensor technologies allow for continuous, decentralized biometric data collection, supporting new models of patient management and remote care.
- Machine learning–driven analytics convert raw patient data into clear recommendations, enabling personalized interventions and supporting adaptable care workflows for institutional buyers.
- Emphasis on user-centric design results in more intuitive interfaces and extended battery life, increasing reliability for both enterprise procurement and individual adopters.
- Strategic collaborations between manufacturers and healthcare providers have streamlined the integration of smart ring data systems, enhancing workflow efficiency and care coordination.
- Successfully entering new markets demands awareness of variable regulatory landscapes, evolving reimbursement protocols, and regional health trends to ensure long-term adoption and compliance.
Tariff Impact and Supply Chain Adaptations
Shifts in tariff structures on medical wearable imports have influenced manufacturers to strengthen relationships with regional component suppliers and enhance logistics processes. These strategies stabilize product availability and keep delivery times responsive. Additionally, modular product architectures allow for production flexibility, helping organizations manage variant design without escalating costs and compete effectively within fluctuating global trade conditions.
Methodology & Data Sources
This report synthesizes quantitative and qualitative research, grounding findings in peer-reviewed articles and regulatory documentation. Primary interviews with product professionals, clinical experts, and procurement leaders add practical perspective. Case study analysis illustrates actual uptake and performance of smart ring technologies in healthcare environments.
Why This Report Matters
- Enables informed strategic decisions by providing market intelligence tailored for healthcare executives in a dynamic medical smart rings sector.
- Supports robust commercial and geographic strategies through detailed segmentation and timely regulatory insights.
- Prepares organizations to anticipate technology, policy, and supply chain shifts to maintain operational resilience and innovation focus.
Conclusion
Medical smart rings are redefining healthcare delivery through adaptive technology and connected care systems. Their versatile capabilities position industry leaders to drive efficient innovation and achieve integration across a fast-evolving digital health landscape.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Medical Smart Rings market report include:- BioRing Technologies Inc
- Circular Life Technologies Inc.
- Harvey Norman Stores Pty Limited
- Infinity Ring Systems Ltd
- LifeRing Tech Solutions Ltd.
- McLear Limited
- Motiv, Inc
- NeoRing Technologies Ltd.
- ORII Labs Ltd. by Crown Laboratories
- Oura Health Oy
- PulseRing, Inc.
- QikRing Innovations Ltd.
- RingConn Technologies Inc.
- Ringly, Inc
- SenseRing, Inc.
- SyncRing Technologies, Inc.
- The NFC Ring Company, LLC
- Token, Inc
- VitaRing Medical Devices, Inc.
- WearX Innovations Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 340.41 Million |
| Forecasted Market Value ( USD | $ 525.55 Million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |