Speak directly to the analyst to clarify any post sales queries you may have.
The radioembolization therapy market is experiencing strategic transformation as stakeholders leverage technological advances and adapt to emerging clinical needs. Executives require up-to-date intelligence to align with regulatory standards and sector best practices while seizing new business opportunities in interventional oncology.
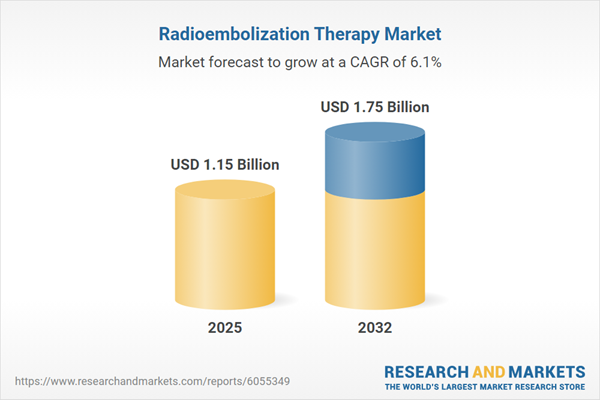
Market Snapshot: Radioembolization Therapy Market Size and Growth Outlook
The radioembolization therapy market is projected to expand from USD 1.09 billion in 2024 to USD 1.15 billion in 2025, anticipating a reach of USD 1.75 billion by 2032, with a compound annual growth rate (CAGR) of 6.05%.
This growth is driven by increasing adoption of radioembolization as supporting clinical evidence continues to build and integration into mainstream oncology pathways progresses. Executives benefit from market insights that pinpoint critical growth factors, potential risks, and areas of competitive advantage.Scope & Segmentation
- Component: Catheters, nuclear medicine systems, X-ray systems, and radioactive microspheres are central to procedural efficiency and safety across facilities.
- Treatment Category: Comprises both curative and palliative radioembolization approaches, enabling providers to tailor therapy for varying cancer presentations.
- Tumor Type: Solutions address both primary and metastatic liver cancers, supporting patient care across a spectrum of indications and extending clinical value.
- Application Procedure: Indications include management of complex liver tumors, with lobar and segmental therapy techniques enabling more precise and personalized treatment.
- End-User: Hospitals, ambulatory surgical centers, and cancer research institutes utilize radioembolization therapy, facilitating both inpatient and outpatient care delivery.
- Region: Coverage includes North and South America (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), and Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan), ensuring strategic insights for both established and growth-stage regions.
- Company Coverage: Industry presence and advancement are backed by leaders such as Cook Medical Inc., Boston Scientific Corporation, Bayer AG, Becton Dickinson & Company, Elekta AB, Fortis Healthcare Limited, Hamilton Health Sciences, Mayo Clinic Health System, Merit Medical Systems, Inc., Nordion Inc., Northwestern Memorial HealthCare, Radiological Society of North America, Inc., Siemens AG, Sirtex Medical Pty Ltd, Stryker Corporation, Terumo Corporation, United HealthCare Services, Inc., and Medtronic plc.
Key Takeaways: Strategic Insights for Decision-Makers
- Radioembolization delivers focused therapy for liver cancers by restricting radiation exposure, safeguarding healthy tissues during intervention.
- Consistent refinement of catheter technology and imaging platforms enhances treatment precision and supports individualized dosing strategies.
- Integrated collaboration amongst radiology, oncology, and nuclear medicine teams is advancing standardization in care, promoting adoption of proven clinical pathways.
- Supply chain management leaders are prioritizing local partnerships and robust logistics to address challenges in distribution and potential market shocks.
- Asia-Pacific markets seek solutions emphasizing scalability, cost-efficiency, and workforce development, while the Americas emphasize procedural specialization and financial innovation.
- Top sector participants continue to set industry benchmarks, broaden solution portfolios, and focus on agreements that deliver sustained value amid sector shifts.
Tariff Impact: Navigating Cost Fluctuations and Supply Chain Resilience
With the introduction of new United States tariffs in 2025, manufacturers are optimizing sourcing and diversifying supply networks to mitigate rising input costs. Healthcare systems are intensifying supplier evaluation and expanding their choice of materials, which is fueling investment into domestic manufacturing capacity. These strategies support continuity of supply for radioembolization therapy products and bolster regional autonomy.
Methodology & Data Sources
This analysis reflects both qualitative and quantitative research, including interviews with executives and clinicians across radiology, oncology, and nuclear medicine sectors. Findings are validated with recent literature, regulatory updates, and patent landscape reviews. In-depth segmentation and supply chain mapping are also applied for a comprehensive market view.
Why This Report Matters
- Delivers senior management with actionable forecasts and strategic market scenarios to guide investment and supplier decisions.
- Offers detailed segmentation and contextually relevant insights to support market entry strategies, expansion, or competitive repositioning.
- Enables data-driven planning and operational flexibility in navigating changes in reimbursement, clinical practice, and regional demand.
Conclusion
To succeed in the dynamic radioembolization therapy market, leaders must prioritize adaptable, evidence-informed strategies. Comprehensive insights provide the foundation for achieving sustainable clinical and commercial progress.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Radioembolization Therapy Market report include:- Cook Medical Inc.
- Boston Scientific Corporation
- Bayer AG
- Becton Dickinson & Company
- Elekta AB
- Fortis Healthcare Limited
- Hamilton Health Sciences
- Mayo Clinic Health System
- Merit Medical Systems, Inc.
- Nordion Inc.
- Northwestern Memorial HealthCare.
- Radiological Society of North America, Inc.
- Siemens AG
- Sirtex Medical Pty Ltd
- Stryker Corporation
- Terumo Corporation
- United HealthCare Services, Inc
- Medtronic plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.15 Billion |
| Forecasted Market Value ( USD | $ 1.75 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |