Speak directly to the analyst to clarify any post sales queries you may have.
Exploring the Complex Pathophysiology and Clinical Imperatives of 3-Kinase Delta Syndrome to Inform Stakeholder Strategies Across the Healthcare Continuum
3-Kinase Delta Syndrome stands at the intersection of genetic mutation and immune dysregulation, presenting a multifaceted challenge for clinicians and researchers alike. Characterized by abnormal activity of the PI3Kδ enzyme, this syndrome manifests through recurrent infections, lymphoproliferation, and a heightened risk of malignancies. Initial clinical presentations often include chronic respiratory issues and impaired antibody responses, underscoring the necessity for early recognition. Moreover, advancements in next-generation sequencing have illuminated the diverse mutation profiles underlying the disorder, revealing both gain-of-function and loss-of-function variants that influence disease trajectory.Furthermore, the rarity of 3-Kinase Delta Syndrome has historically delayed comprehensive understanding, necessitating collaborative research networks and patient registries to consolidate clinical data. In recent years, diagnostic guidelines have evolved, encompassing immunophenotyping, functional assays, and genomic analyses to ensure accurate subtype classification. Additionally, increasing awareness among healthcare professionals has fostered more timely referrals to specialized immunology centers.
Consequently, stakeholders across academia, pharmaceutical development, and patient advocacy are uniting around a shared goal: translating the expanding body of molecular insights into targeted therapeutic strategies. As we explore the transformative shifts shaping this field, it becomes clear that integrated approaches-combining precision diagnostics, tailored treatments, and multidisciplinary care pathways-are essential to improving outcomes for individuals affected by this complex immunodeficiency.
Identifying Transformative Shifts in Diagnostic, Therapeutic, and Regulatory Landscapes Shaping the Future Management of 3-Kinase Delta Syndrome
The landscape of 3-Kinase Delta Syndrome management is undergoing a profound transformation driven by converging innovations in diagnostics, therapeutics, and policy frameworks. Initially centered on broad-spectrum immunosuppression and symptomatic care, the field has shifted toward precision interventions that modulate the PI3Kδ pathway with unprecedented specificity. Targeted inhibitors, bolstered by insights into enzyme structure and mutation heterogeneity, are redefining treatment paradigms and reducing off-target effects.Meanwhile, parallel advances in digital health platforms have enabled remote monitoring of immunoglobulin levels and infection markers, enhancing continuity of care and patient adherence. Telemedicine consultations now complement in-clinic evaluations, allowing for dynamic treatment adjustments based on real-time data. In addition, regulatory bodies have begun issuing expedited designations for novel agents addressing rare immunodeficiencies, streamlining the path from clinical trial to clinical practice.
Moreover, the integration of machine learning algorithms to analyze longitudinal patient data is uncovering predictive biomarkers of treatment response, guiding clinician decision-making and trial design. Collaborative frameworks among academic centers, biotech startups, and patient advocacy groups are further accelerating therapeutic discovery. As a result, the collective momentum in research investment, regulatory alignment, and technological deployment is reshaping the future of 3-Kinase Delta Syndrome care, driving toward more durable remissions and improved quality of life.
Assessing the Cumulative Impact of 2025 United States Tariff Policies on Therapeutic Supply Chains and Treatment Accessibility for 3-Kinase Delta Syndrome
United States tariff adjustments slated for 2025 will exert a cumulative influence on the supply chains underpinning essential therapies for immunodeficiency syndromes. In particular, tariffs on biologic active pharmaceutical ingredients and vector components for gene therapies may elevate procurement costs and create logistical complexities for contract manufacturers. Consequently, pharmaceutical firms are reevaluating their global sourcing strategies, exploring nearshoring opportunities to mitigate tariff impacts and maintain a steady flow of critical inputs.Concurrently, changes to import duties on specialized medical devices and cold-chain equipment could translate into increased operational expenses for clinics administering immunoglobulin infusions and hematopoietic stem cell transplants. As a result, health systems may face heightened budgetary pressures, prompting negotiations with suppliers to secure cost-containment measures. In response, some organizations are proactively investing in domestic production capacities and strategic stockpiling to ensure uninterrupted patient access.
Furthermore, the 2025 tariff landscape underscores the importance of regulatory engagement. Companies are collaborating with trade associations and government stakeholders to advocate for exemptions on life-saving therapies, highlighting the public health implications of supply disruptions. By aligning commercial strategies with policy advocacy and supply chain resilience planning, stakeholders can navigate the evolving tariff environment while safeguarding treatment accessibility for patients with 3-Kinase Delta Syndrome.
Deriving Actionable Insights from Multifaceted Segmentations Spanning Treatment Modalities, Disease Stages, Genetic Profiles, and Care Settings
A nuanced understanding of patient subgroups is essential for optimizing therapeutic impact in 3-Kinase Delta Syndrome. Based on treatment modalities, antibiotic prophylaxis remains a frontline approach to reduce infection frequency, whereas hematopoietic stem cell transplantation offers the potential for long-term disease modification. Immunoglobulin replacement therapy serves as a cornerstone for passive immunity, and immunosuppressive regimens address underlying autoinflammatory manifestations. Each modality warrants careful consideration of risk-benefit profiles, particularly in light of emerging targeted inhibitors that may complement or supersede existing protocols.Equally important is recognizing differences in drug delivery, as injectables enable precise dosing of biologic agents, while oral formulations support patient convenience and adherence. In early disease stages, rapid intervention with targeted therapies can forestall irreversible organ damage, whereas advanced stage presentations often necessitate combination approaches to manage lymphoproliferation and immune dysregulation. Achieving complete or partial remission requires vigilant monitoring, adaptive treatment adjustments, and a coordinated care framework to sustain durable responses.
Genetic mutation profiling further refines patient stratification, distinguishing gain-of-function variants-such as E1021K and E162K-that drive hyperactivation from loss-of-function alterations involving splice site or truncating mutations. These molecular insights inform both prognosis and therapeutic selection. Meanwhile, the settings in which patients receive care-from home-based infusion services to hospital units, outpatient clinics, and specialized centers-shape pathways for monitoring, education, and support. Finally, tailoring strategies to adult, geriatric, and pediatric populations ensures that age-specific physiological considerations guide dosing, safety monitoring, and long-term outcome planning.
Unraveling Regional Dynamics and Healthcare Infrastructure Variances Across the Americas, Europe, Middle East & Africa, and Asia-Pacific Markets
Regional healthcare ecosystems exhibit marked differences in infrastructure, reimbursement pathways, and regulatory frameworks that shape patient access to novel immunodeficiency treatments. In the Americas, robust clinical trial networks and supportive regulatory incentives have accelerated the approval of targeted inhibitors and gene-based therapies. This region's emphasis on patient registries and real-world evidence generation fosters iterative improvements in treatment protocols and payer engagement strategies.By contrast, the Europe, Middle East & Africa landscape is characterized by heterogeneous market dynamics, where central regulatory authorities coexist with country-specific reimbursement processes. Stakeholders must navigate varying approval timelines and health technology assessment requirements, while collaborative procurement initiatives are gaining traction to enhance affordability and distribution in under-served areas.
In Asia-Pacific, emerging economies are investing heavily in biotechnology innovation and local manufacturing capabilities to reduce dependency on imports. Government initiatives aimed at strengthening rare disease registries and subsidizing high-cost therapies are gradually improving access, although disparities persist across urban and rural settings. As a result, multinational pharmaceutical companies are forging regional partnerships and licensing agreements to tailor their go-to-market strategies to the unique regulatory and economic contours of each market.
Highlighting Strategic Collaborations and Innovation Portfolios of Leading Biopharmaceutical Companies in the 3-Kinase Delta Syndrome Arena
The competitive landscape for 3-Kinase Delta Syndrome therapies features a blend of established pharmaceutical giants and agile biotech innovators. Leading companies are forging strategic collaborations to bolster their pipelines, with several pursuing next-generation PI3Kδ inhibitors that promise enhanced selectivity and safety profiles. Concurrently, gene therapy ventures are advancing preclinical programs aimed at correcting pathogenic mutations, underpinned by platform technologies that streamline vector design and delivery.Partnership agreements between large-cap firms and niche developers are catalyzing access to cutting-edge modalities. These alliances often encompass co-development of companion diagnostics, ensuring that therapeutic interventions align with specific genetic mutation profiles. Mergers and acquisitions continue to reshape the landscape, as companies seek scale and complementary capabilities to strengthen their positions in this specialized space.
Moreover, investment in manufacturing capacity for both biologic agents and viral vector platforms reflects a strategic intent to secure supply chain robustness. By integrating clinical development with scalable production infrastructures, organizations can accelerate time-to-market while maintaining stringent quality standards. As competition intensifies, the alignment of R&D priorities with commercial execution will be decisive in capturing the evolving opportunities presented by 3-Kinase Delta Syndrome treatment innovation.
Formulating Actionable Recommendations for Industry Leaders to Enhance Patient Outcomes, Accelerate Innovation, and Optimize Market Positioning
Industry leaders must adopt a proactive, patient-centric approach to navigate the complexities of 3-Kinase Delta Syndrome management. First, scaling precision diagnostic capabilities through expanded genetic testing infrastructure will be critical to identifying candidates for targeted therapies and clinical trials. Second, forging deeper partnerships with payers and healthcare systems can streamline reimbursement pathways and mitigate access barriers for high-cost treatments.In addition, investing in real-world evidence initiatives will generate robust data to substantiate long-term efficacy and safety, thereby reinforcing market uptake and payer confidence. Furthermore, diversifying supply chains through strategic nearshoring and vendor partnerships will enhance resilience against external shocks such as tariff fluctuations or raw material shortages.
To sustain innovation momentum, organizations should explore gene editing collaborations that leverage emerging CRISPR-based platforms, while prioritizing patient support programs that address the holistic needs of individuals and caregivers. Finally, embracing digital health tools for remote monitoring and telemedicine consultations will not only improve adherence but also foster a seamless continuum of care across geographies.
Detailing the Robust Research Methodology Integrating Qualitative Interviews, Secondary Data Analysis, and Expert Validation for Report Integrity
This report is grounded in a rigorous research methodology that integrates multiple evidence streams to ensure comprehensiveness and reliability. Initial desk research encompassed a systematic review of peer-reviewed journals, regulatory filings, and conference proceedings to construct a foundational knowledge base. This was complemented by analysis of clinical trial registries and patent landscapes to capture the latest therapeutic innovations and intellectual property trends.To enrich quantitative insights, a series of in-depth interviews were conducted with leading immunologists, clinical trial investigators, regulatory experts, and commercial executives. These qualitative engagements provided firsthand perspectives on diagnostic practices, patient journey challenges, and commercialization dynamics. Subsequently, findings were triangulated through cross-validation workshops, aligning stakeholder viewpoints and resolving discrepancies.
Moreover, secondary data from health technology assessment agencies and publicly available reimbursement databases were examined to map regional access frameworks. All inputs underwent critical appraisal against predefined quality criteria, ensuring that the report's conclusions rest upon a robust and transparent analytical foundation. This multi-method approach delivers actionable intelligence while maintaining methodological integrity.
Synthesizing Key Findings and Strategic Imperatives to Conclude on the Evolving Landscape of 3-Kinase Delta Syndrome Management
In summary, the evolving landscape of 3-Kinase Delta Syndrome is defined by a convergence of molecular insights, therapeutic innovation, and shifting policy frameworks. Enhanced genetic profiling and precision diagnostics have paved the way for targeted inhibitors and potential gene-based cures, while digital health solutions are transforming care delivery and patient engagement. At the same time, geopolitical factors such as tariff reforms underscore the importance of supply chain resilience and strategic policy advocacy.Regional disparities in regulatory pathways and reimbursement systems present both challenges and opportunities for market entry, necessitating tailored strategies that account for local nuances. The competitive environment is characterized by strategic alliances, M&A activity, and manufacturing investments aimed at securing pipeline robustness and supply security. Against this backdrop, industry leaders are called upon to adopt patient-centric models, invest in real-world evidence, and cultivate collaborative networks that accelerate translational research.
Ultimately, the key to advancing care for individuals with 3-Kinase Delta Syndrome lies in the integration of cutting-edge science, adaptive commercial models, and a steadfast commitment to overcoming access barriers. As the field continues to progress, stakeholders who harness these insights will be best positioned to deliver meaningful improvements in patient outcomes and long-term durability of therapeutic regimens.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Treatment
- Antibiotic Prophylaxis
- Hematopoietic Stem Cell Transplant
- Immunoglobulin Replacement Therapy
- Immunosuppressants
- Mode of Administration
- Injectables
- Oral
- Disease Stage
- Advanced Stage
- Early Stage
- Remission
- Complete Remission
- Partial Remission
- Genetic Mutation Profile
- Gain-Of-Function Mutations
- E1021K Variant
- E162K Variant
- Loss-Of-Function Mutations
- Splice Site Mutations
- Truncating Mutations
- Gain-Of-Function Mutations
- End-Use
- Home Care
- Hospitals
- Outpatient Clinics
- Specialty Clinics
- Patient Type
- Adult Patients
- Geriatric Patients
- Pediatric Patients
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AbbVie Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- BeiGene Ltd.
- Genomenon, Inc.
- Gilead Sciences, Inc.
- Incyte Corporation
- Infinity Pharmaceuticals, Inc.
- Kyowa Kirin Co., Ltd
- MEI Pharma, Inc.
- Merck KGaA
- Novartis AG
- Pharming Group N.V.
- Sanofi S.A.
- Secura Bio, Inc.
- TG Therapeutics, Inc.
- Verastem, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this 3-Kinase Delta Syndrome market report include:- AbbVie Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- BeiGene Ltd.
- Genomenon, Inc.
- Gilead Sciences, Inc.
- Incyte Corporation
- Infinity Pharmaceuticals, Inc.
- Kyowa Kirin Co., Ltd
- MEI Pharma, Inc.
- Merck KGaA
- Novartis AG
- Pharming Group N.V.
- Sanofi S.A.
- Secura Bio, Inc.
- TG Therapeutics, Inc.
- Verastem, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
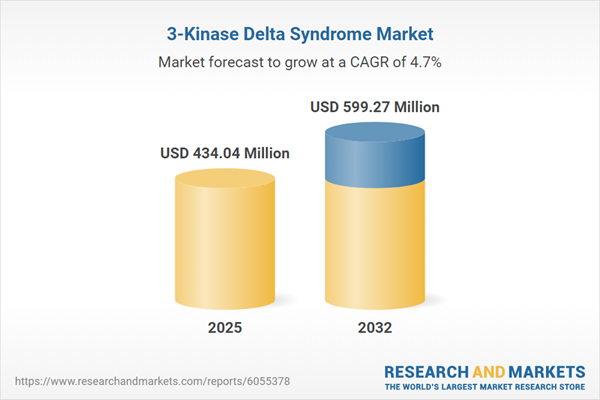
| Estimated Market Value ( USD | $ 434.04 Million |
| Forecasted Market Value ( USD | $ 599.27 Million |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |