Speak directly to the analyst to clarify any post sales queries you may have.
The Biliary Tract Cancers Market is evolving rapidly, shaped by new diagnostic technologies, treatment innovations, and changing regulatory and trade environments. Executive leaders and key stakeholders require precise, actionable intelligence to navigate this transforming landscape and maximize business impact.
Market Snapshot: Growth Drivers and Key Trends
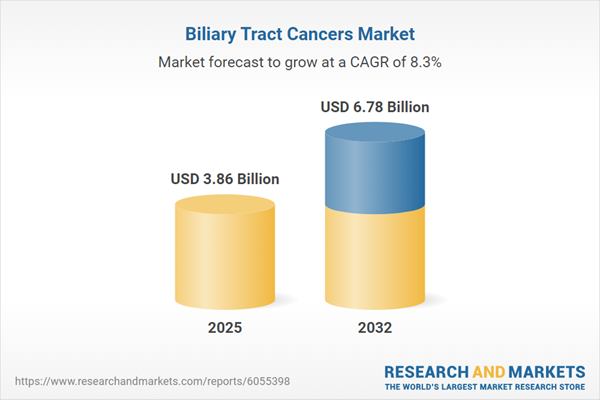
The Biliary Tract Cancers Market grew from USD 3.58 billion in 2024 to USD 3.86 billion in 2025. It is expected to continue growing at a CAGR of 8.30%, reaching USD 6.78 billion by 2032.
Market expansion is fueled by advancements in molecular diagnostics, increasing demand for precision medicine, and the introduction of targeted therapies. Investment in collaborative research and seamless integration of new technologies into care pathways create additional momentum. Shifts in regulations and supply chain factors, partly influenced by global policies, also contribute to continued growth and present both challenges and strategic opportunities for market participants.Scope & Segmentation: In-Depth Coverage Across the Biliary Tract Cancers Market
- Cancer Types: Cholangiocarcinoma, Gallbladder Cancer
- Treatment Types: Chemotherapy, Immunotherapy, Radiation Therapy, Surgery, Targeted Therapy
- End-Users: Academic Institutions, Cancer Research Institutes, Hospitals & Clinics
- Distribution Channels: Offline, Online
- Geographic Coverage: Americas (North America: United States, Canada, Mexico; Latin America: Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (Europe: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland; Middle East: United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel; Africa: South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Companies Analyzed: Agios Pharmaceuticals, Amgen, AstraZeneca, Basilea Pharmaceutica, BeiGene, Bristol-Myers Squibb, Delcath Systems, Eisai, Eli Lilly, Exelixis, F. Hoffmann-La Roche, Incyte, LES LABORATOIRES SERVIER, Merck & Co., Novartis, Pfizer, Sanofi, TAIHO PHARMACEUTICAL, TransThera Sciences, Zymeworks
Biliary Tract Cancers Market: Key Takeaways for Decision Makers
- Next generation sequencing and biomarker-driven trials are shaping personalized therapy strategies and improving patient stratification.
- Targeted therapies and immune checkpoint inhibitors are establishing new care standards, especially in select subpopulations with actionable mutations.
- Artificial intelligence and machine learning enhance diagnostic workflows and prognostic accuracy, driving improvements in treatment planning.
- Surgical interventions remain critical, yet integration with advanced therapies and minimally invasive techniques is optimizing patient outcomes.
- Partnerships between leading pharmaceutical firms, biotech innovators, and academic centers are accelerating clinical development pipelines and expanding access to new treatment options.
- Evolving reimbursement structures, regulatory policies, and industry collaborations will be vital to market access and sustainable innovation.
Tariff Impact: Strategic Considerations for 2025 and Beyond
Recent tariff changes in the United States have affected the cost and availability of key oncology therapeutics and diagnostics. Manufacturers and research organizations have responded by diversifying sourcing strategies, forming new domestic partnerships, and reevaluating global production models. These shifts necessitate careful supply chain risk management and enhance the importance of policy advocacy to maintain unbroken access to life-saving treatments.
Methodology & Data Sources
This report utilizes a multi-phase research model, combining comprehensive reviews of peer-reviewed literature, patent data, clinical trial registries, and policy documents. Expert insights were gained through in-depth interviews with oncologists, researchers, regulatory specialists, and industry leaders. Analytical frameworks—including SWOT, gap analysis, and trend mapping—support robust validation and actionable findings.
Why This Report Matters
- Provides clear, consolidated market intelligence to support strategic planning, investment, and competitive positioning in the biliary tract cancers market.
- Offers segment-level insights to identify emerging opportunities, mitigate risks, and inform decision making for R&D, business development, and policy advocacy.
- Enables benchmarking across regions and competitors, with specific focus on evolving technologies and distribution networks.
Conclusion
Senior decision-makers seeking actionable insight into the biliary tract cancers market will find this report an essential resource for shaping effective strategies. Integrated analysis and robust methodology support decisive action in a fast-evolving therapeutic landscape.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Biliary Tract Cancers market report include:- Agios Pharmaceuticals, Inc.
- Amgen Inc.
- AstraZeneca PLC
- Basilea Pharmaceutica AG
- BeiGene, Ltd.
- Bristol-Myers Squibb Company
- Delcath Systems, Inc.
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- F. Hoffmann-La Roche Ltd
- Incyte Corporation
- LES LABORATOIRES SERVIER
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- TAIHO PHARMACEUTICAL CO., LTD.
- TransThera Sciences (Nanjing), Inc.
- Zymeworks Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 3.86 Billion |
| Forecasted Market Value ( USD | $ 6.78 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |