Speak directly to the analyst to clarify any post sales queries you may have.
Senior decision-makers engaged in the human metapneumovirus treatment market operate within a rapidly evolving environment shaped by accelerated clinical advancements, adaptive regulations, and patient-focused care models. Success in this sector relies on strategic flexibility, operational responsiveness, and proactive alignment with technological progress.
Market Snapshot: Human Metapneumovirus Treatment Market Overview
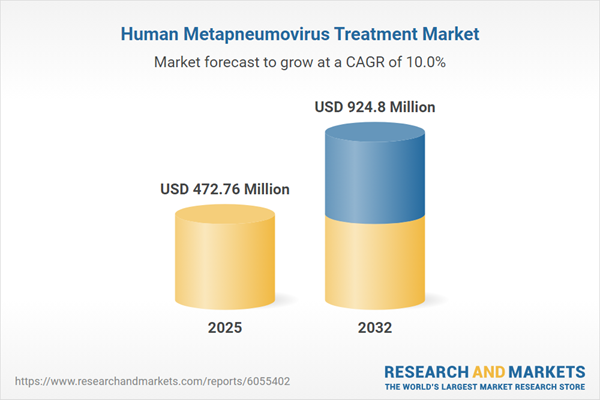
The human metapneumovirus treatment market is experiencing robust expansion, having grown from USD 432.49 million in 2024 to USD 472.76 million in 2025, and projected to achieve a compound annual growth rate (CAGR) of 9.96%, ultimately reaching USD 924.80 million by 2032. This momentum is propelled by increasing uptake of advanced antiviral therapies, enhanced supportive care options, and widespread adoption of technology-enabled healthcare delivery. Healthcare providers are responding to unmet needs, especially among at-risk populations such as infants, older adults, and those with compromised immunity. The reliability of supply chains, maturity of healthcare infrastructure, and consistent reimbursement models across key geographies further strengthen market growth.
Scope & Segmentation: Higher-Value Opportunities
- Genotypes: Genotype A (A1, A2) and genotype B (B1, B2) create distinct opportunities for targeted therapies and inform research priorities, allowing treatment strategies to adapt to evolving viral variations.
- Treatment Type: The segment encompasses antiviral agents like intravenous immunoglobulin and ribavirin, as well as pipeline therapies and critical supportive care including fluid management, oxygen supplementation, pain relief, anti-pyretics, antibiotics, bronchodilators, and corticosteroids.
- Patient Type: The market addresses adults, geriatrics, and pediatric patients, prioritizing tailored protocols for infants and young children with a focus on precise dosing and age-specific management guidelines.
- Route of Administration: Inhalation, oral, and parenteral treatment options equip clinicians to align administration with patient complexity and individual risk profiles effectively.
- End User: Solutions are deployed across clinics, hospitals, and homecare settings, supporting organizational readiness and broadening patient access to appropriate care environments.
- Distribution Channel: Hospital, retail, and online pharmacies facilitate streamlined access for clinicians and patients, enabling continuity of care in both digital and physical channels.
- Regional Scope: Coverage includes the Americas, Europe, Middle East & Africa, and Asia-Pacific, with notable regional contributions from the United States, Brazil, United Kingdom, Germany, India, and China, representing crucial centers of market expansion and demand.
- Key Players: AstraZeneca PLC, Creative Biolabs Inc., Alnylam Pharmaceuticals, Creative Diagnostics, Hoffmann-La Roche Ltd, Merck KGaA, Moderna Inc., Pfizer Inc., Sanofi-aventis Group, Shionogi & Co., The Native Antigen Company, Thermo Fisher Scientific Inc., and ViceBio Limited collectively drive innovation and reinforce competitive dynamics through active research and product development pipelines.
Key Takeaways for Strategic Decision-Makers
- Precision medicine is advancing as novel targeted antiviral therapies become integral to individualized patient care, especially among high-priority segments.
- Integration of digital health tools, such as telemedicine and remote patient monitoring, is improving overall care efficiency and enabling earlier risk identification among vulnerable groups.
- Diverse viral genotypes and patient needs are prompting versatile therapy development and informing new clinical trial designs for broader treatment efficacy.
- Strategic collaborations between pharmaceutical companies, biotechnology innovators, and academic organizations are catalyzing therapy development and streamlining the introduction of novel treatment options.
- Changes in payer requirements and regulatory frameworks are increasing the emphasis on real-world evidence and clinical outcomes to optimize reimbursement and access strategies.
- Investments in continuous provider education and updated training are essential for rapid adoption of emerging clinical protocols and for promoting standardized care across all delivery environments.
Tariff Impact: Evolving Trade and Supply Chain Dynamics
Recent changes in U.S. tariff policies have influenced manufacturing and procurement within the human metapneumovirus treatment market. Stakeholders are adapting by prioritizing nearshoring and reinforcing supply chain resilience, which introduces additional considerations for pricing, reimbursement optimization, and navigating regulatory compliance. Developing specialized expertise in these areas is becoming increasingly important as the market contends with legislative shifts and operational complexities.
Methodology & Data Sources
This analysis combines direct interviews with industry opinion leaders and frontline clinicians, peer-reviewed academic literature, regulatory documentation, and clinical trial results. Each data source undergoes rigorous validation and impartial peer review to secure the quality and reliability of insights provided.
Why This Report Matters
- Empowers executive teams to anticipate trends in therapy innovation, regulatory shifts, and technology adoption within the human metapneumovirus treatment market.
- Delivers actionable segmentation and regional perspectives, supporting superior market entry decisions, strategic partnerships, and optimal allocation of resources.
- Equips stakeholders with authoritative, up-to-date intelligence essential for risk mitigation, capital planning, and tracking performance in a complex healthcare landscape.
Conclusion
Aligning with clinical progress, regulatory evolution, and untapped opportunities in the human metapneumovirus treatment market enables organizations to enhance patient outcomes and realize sustainable business value.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Human Metapneumovirus Treatment Market report include:- AstraZeneca PLC
- Creative Biolabs, Inc.
- Alnylam Pharmaceuticals
- Creative Diagnostics
- Hoffmann-La Roche Ltd
- Merck KGaA
- Moderna, Inc.
- Pfizer Inc.
- Sanofi-aventis Group
- Shionogi & Co.
- The Native Antigen Company
- Thermo Fisher Scientific Inc.
- ViceBio Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 472.76 Million |
| Forecasted Market Value ( USD | $ 924.8 Million |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |