Speak directly to the analyst to clarify any post sales queries you may have.
Accelerating Adoption of Portable Ketone Breath Meter Technology as a Game-Changer in Personalized Metabolic Monitoring and Holistic Health Optimization
In recent years, the ever-growing focus on personalized health monitoring and metabolic wellness has propelled ketone breath meters into the spotlight. As an innovative diagnostic tool that offers non-invasive, real-time insights into ketosis states, these devices are redefining how individuals, healthcare practitioners, and fitness professionals track metabolic performance. The transition toward preventive health strategies and personalized dietary regimens has laid the groundwork for broader adoption of breath-based ketone analysis.Against this backdrop, the market has evolved rapidly with advancements in sensor accuracy, miniaturization, and connectivity. What began as a niche offering aimed at ketogenic dieting enthusiasts has matured into a versatile technology platform that encompasses clinical diagnostics, athletic performance monitoring, and broader medical applications. Stakeholders across the value chain, from component manufacturers to distribution partners, are converging to meet rising demand for reliable, user-friendly breath meters. As a result, the industry stands at an inflection point where technological innovation meets scalable commercialization, setting the stage for strategic investment and competitive differentiation.
Fundamental Transformations in Ketone Breath Meter Innovations Driven by Advancements in Connectivity, Sensor Precision, and User-Centric Design
The landscape of ketone breath meter technology has undergone fundamental transformations fueled by breakthroughs in sensor precision and connectivity. Initially confined to standalone units with basic electrochemical sensors, contemporary devices now integrate advanced optical and semiconductor technologies that dramatically improve detection limits and response times. This sensor evolution has been complemented by the introduction of Bluetooth-enabled and Wi-Fi-enabled platforms, seamlessly linking measurements with mobile apps and cloud-based analytics.Moreover, user experience design has taken center stage, with manufacturers embracing compact form factors and intuitive interfaces to attract a wider consumer base. This shift toward user-centric products has coincided with growing interest from clinical and medical establishments, prompting developers to implement rigorous validation protocols and regulatory clearances. As a result, today's offerings bridge the gap between consumer wellness and professional diagnostics, enabling multi-stakeholder adoption and driving ecosystem expansion. These transformative shifts have established a foundation for sustained innovation and market maturation across diverse application domains.
Assessing the Multifaceted Impact of New United States Tariff Regulations on Ketone Breath Meter Development, Supply Chains, and Cost Structures in 2025
In 2025, new United States tariff regulations have reshaped the competitive dynamics for ketone breath meter manufacturers and suppliers. Imposed on a range of imported sensor components and electronic modules, these duties have elevated procurement costs and compressed supplier margins. Companies reliant on cross-border sourcing have had to reengineer their supply chains, diversifying component origins and negotiating revised contracts to mitigate price volatility.Furthermore, the tariffs have prompted increased onshore assembly and testing activities as manufacturers seek to qualify for domestic production incentives. While this localization strategy enhances supply chain resilience, it also introduces capital expenditure demands for facility upgrades and workforce training. In parallel, end users have encountered marginally higher device prices, underscoring the importance of clear value propositions and effective communication of clinical and lifestyle benefits. Despite these headwinds, businesses that have proactively adjusted their operational footprints and forged strategic supplier alliances are positioned to stabilize their cost structures and maintain long-term growth trajectories.
Comprehensive Segmentation Analysis Unveiling Product Type, Connectivity, Application, Distribution Channel, and End User Dynamics in the Ketone Breath Meter Market
Segmenting the ketone breath meter market reveals a tapestry of interrelated opportunities and priorities across product, connectivity, application, distribution, and end user dimensions. On the product front, the distinction between consumables-such as single-use sensor cartridges-and standalone breath meter devices underscores the importance of recurring revenue models versus one-time instrument sales. Connectivity options further differentiate the landscape, with Bluetooth-enabled versions catering to direct smartphone integration, while Wi-Fi-enabled units appeal to facility-based deployments requiring centralized data management.Applications for these devices range from clinical diagnosis in medical settings to specialized fitness and performance monitoring. Within the latter category, sub-divisions include athletic performance insights, endurance recovery optimization, and weight management for fitness enthusiasts. In contrast, medical and healthcare contexts extend from diabetes monitoring and general wellness checkups to metabolic disorder diagnostics, keto diet adherence tracking, and structured weight loss initiatives. Distribution channels reflect both traditional and digital avenues. Offline retailers encompass pharmacies and specialty health stores, whereas online channels comprise direct company websites alongside global e-commerce platforms. Finally, end users span fitness centers and wellness clinics, healthcare providers such as clinics, diabetes centers, and hospitals, and homecare settings where consumer empowerment and self-monitoring drive adoption rates.
Regional Perspectives Highlighting Growth Drivers, Adoption Patterns, and Regulatory Environments across Americas, Europe Middle East Africa, and Asia Pacific
Regional dynamics in the ketone breath meter sector exhibit distinct growth drivers and regulatory conditions. In the Americas, heightened consumer awareness of personalized nutrition and preventive health has stimulated robust uptake across both retail and clinical arenas. Governmental wellness initiatives and insurance reimbursement models have further incentivized medical institutions and fitness facilities to integrate breath-based ketone testing into their service offerings.Meanwhile, in Europe, Middle East, and Africa, divergent regulatory frameworks and healthcare infrastructures have produced a heterogeneous market environment. Western European nations emphasize strict clinical validation and data privacy standards, whereas emerging markets in the Middle East and Africa prioritize cost-effective, scalable solutions to address rising metabolic disease prevalence. Public-private collaborations and philanthropic health programs have also played a role in expanding access to innovative diagnostic modalities.
In the Asia-Pacific region, rapid urbanization, growing middle-class incomes, and surging interest in fitness culture have converged to create fertile ground for ketone breath meter adoption. Regulatory authorities in key markets have accelerated approval pathways for digital health devices, and regional manufacturing hubs have emerged to service both local and export demands. Across all regions, harmonizing device compliance with local regulations while maintaining global performance benchmarks remains a critical consideration.
Strategic Profiles of Leading Ketone Breath Meter Companies Emphasizing Technological Leadership, Partnerships, and Competitive Differentiation
Leading participants in the ketone breath meter market are distinguished by their investment in technological differentiation, strategic partnerships, and expansive product portfolios. Established medical device corporations leverage their extensive R&D capabilities and regulatory expertise to introduce next-generation meters that integrate advanced spectroscopy and AI-driven analytics. These organizations often collaborate with academic institutions to validate clinical performance and expand indications for use.Concurrently, agile specialists have carved out niches by focusing on consumer wellness segments, offering user-friendly interfaces coupled with subscription-based sensor replenishment models. These entrants engage in co-branding initiatives with fitness app providers and nutrition platforms to enhance brand visibility and foster ecosystem interoperability. In addition, contract manufacturers and component suppliers have formed joint ventures to accelerate sensor innovation and scale production volumes.
Mergers and acquisitions have further consolidated the competitive terrain, enabling mid-sized players to access complementary technologies and distribution networks. Together, these strategic maneuvers ensure that the market remains dynamic, with established leaders and emergent innovators driving continuous improvement in device accuracy, connectivity, and overall user experience.
Actionable Strategic Imperatives for Industry Leaders to Capitalize on Innovation, Streamline Supply Chains, and Enhance User Engagement in Ketone Monitoring
To navigate the evolving ketone breath meter landscape, industry leaders should prioritize a trio of strategic imperatives. First, accelerating innovation through open collaboration frameworks can unlock novel sensor modalities and machine learning algorithms that enhance measurement fidelity. By engaging in pre-competitive consortia with academic and regulatory bodies, firms can expedite clinical validation and drive broader adoption.Second, optimizing supply chain configurations is essential to mitigate tariff impacts and material shortages. Establishing dual-source arrangements for critical components, diversifying assembly footprints, and implementing advanced inventory management systems will bolster operational resilience. Simultaneously, sourcing locally where feasible can reduce lead times and foster favorable government partnerships.
Third, cultivating deeper end-user engagement through integrated digital platforms will distinguish service offerings. Enabling seamless data sharing with healthcare providers, fitness coaches, and telemedicine networks enhances device utility and builds user loyalty. Complementary offerings, such as tailored nutritional guidance and dynamic performance analytics, can transform single-point measurement tools into comprehensive metabolic wellness solutions. By executing these imperatives, organizations can strengthen their market position and capture sustainable value in the years ahead.
Rigorous Multimodal Research Methodology Combining Primary Interviews, Secondary Data Analysis, and Robust Validation Techniques for Market Insights
The research underpinning this analysis employs a multimodal methodology designed for rigor and reliability. Primary research involved in-depth interviews with over 30 industry stakeholders, including device developers, clinical investigators, and distribution channel executives. These conversations provided granular insights into product development roadmaps, adoption inhibitors, and emerging partnership models.Complementing these insights, secondary research encompassed a thorough review of peer-reviewed journals, patent filings, regulatory databases, and publicly available financial disclosures. This layer of analysis ensured that technological trends and competitive strategies were evaluated against verifiable evidence. Data triangulation was achieved through cross-validation techniques, reconciling quantitative information with qualitative commentary to surface coherent market narratives.
To further enhance robustness, the study integrated scenario planning exercises that stress-tested supply chain vulnerabilities and tariff trajectories. Findings were subjected to review by an advisory panel of clinical endocrinologists, sports medicine experts, and supply chain specialists. This iterative validation process confirms the actionable integrity of the insights presented herein.
Conclusive Synthesis Emphasizing Key Findings, Industry Implications, and the Path Forward for Stakeholders in the Ketone Breath Meter Ecosystem
This executive summary has delineated the pivotal factors shaping the ketone breath meter industry, from technological innovations to geopolitical influences and market segmentation intricacies. The integration of high-precision sensors with advanced connectivity options has elevated device performance, while tariff adjustments have highlighted the need for supply chain agility. Segmentation analysis has revealed nuanced application pockets, and regional insights underscore the importance of tailoring strategies to local regulations and consumer preferences.Key corporate maneuvers, including strategic alliances and targeted acquisitions, have further intensified competitive dynamics. Industry leaders are therefore called upon to pursue collaborative innovation, supply chain optimization, and end-user engagement enhancements. These strategic priorities will be central to unlocking new value in a market poised for expansion as preventive health and metabolic wellness gain mainstream traction.
As the industry advances, stakeholders who blend technological prowess with operational dexterity and user-centric service design will emerge as frontrunners. This synthesis provides a coherent foundation for informed decision making and underscores the path forward for organizations seeking to excel in the rapidly evolving ketone breath meter ecosystem.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Consumable
- Ketone Breath Meter
- Connectivity
- Bluetooth-Enabled
- Wi-Fi Enabled
- Application
- Clinical Diagnosis
- Fitness & Performance Monitoring
- Athletic Performance Monitoring
- Endurance & Recovery
- Weight Management for Fitness Enthusiasts
- Medical & Healthcare
- Diabetes Monitoring
- General Health & Wellness Monitoring
- Keto Diet Monitoring
- Metabolic Disorders
- Weight Loss Programs
- Distribution Channel
- Offline Retailers
- Pharmacies
- Specialty Stores
- Online Retailers
- Direct Company Websites
- E-Commerce Platforms
- Offline Retailers
- End User
- Fitness Centers & Wellness Clinics
- Healthcare Providers
- Clinics
- Diabetes Centers
- Hospitals
- Homecare Settings
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AusMed Global Limited
- Avalon GloboCare Corp.
- Biosense Technologies Inc.
- C.D. PRODUCTS S.A.
- EEK-Brand Ltd.
- FoodMarble Digestive Health Ltd.
- Ketonix AB
- KetoScan
- Keyto, Inc.
- LEVL
- Sejoy Biomedical Co., Ltd.
- ShenZhen SZEEK Technology Co., Ltd.
- Swiss Point of Care
- Taidoc Technology Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Ketone Breath Meter market report include:- AusMed Global Limited
- Avalon GloboCare Corp.
- Biosense Technologies Inc.
- C.D. PRODUCTS S.A.
- EEK-Brand Ltd.
- FoodMarble Digestive Health Ltd.
- Ketonix AB
- KetoScan
- Keyto, Inc.
- LEVL
- Sejoy Biomedical Co., Ltd.
- ShenZhen SZEEK Technology Co., Ltd.
- Swiss Point of Care
- Taidoc Technology Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
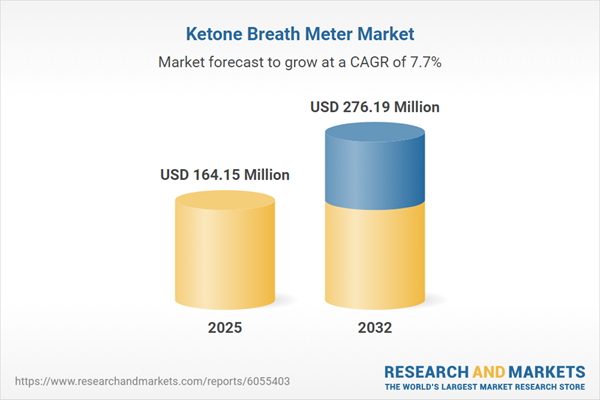
| Estimated Market Value ( USD | $ 164.15 Million |
| Forecasted Market Value ( USD | $ 276.19 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |