Speak directly to the analyst to clarify any post sales queries you may have.
The in vitro micro electrode array market is witnessing sustained momentum as advanced electrophysiological research tools become integral to neuroscience and drug discovery programs. Senior industry leaders are increasingly leveraging this technology for enhanced cellular analysis and reliable preclinical screening.
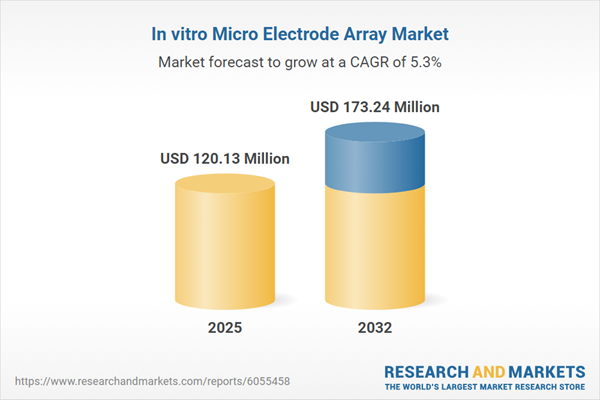
Market Snapshot: In Vitro Micro Electrode Array Market
In 2024, the in vitro micro electrode array market was valued at USD 114.45 million. The sector is set to grow steadily, reaching USD 120.13 million by 2025, with continued expansion anticipated at a CAGR of 5.31%. By 2032, projections indicate a value of USD 173.24 million. Growth is driven by the increasing demand for high-resolution data in translational research and pharmaceutical applications.
Scope & Segmentation
This report delivers a comprehensive analysis of the in vitro micro electrode array market across all core segments, end-users, geographies, and technologies, capturing the evolving needs of diverse stakeholders.
- Product: Consumables, Cleaning & Maintenance Kits, MEA Plates/Chips, Reagents & Media, Instruments, MEA Recording Systems, MEA Stimulation Systems
- Type: Multiwell Microelectrode Arrays, Single-Well Microelectrode Arrays
- Application: Disease Modeling, Drug Discovery, Neuroscience, Tissue Engineering, Toxicology Testing
- End-User: Academic & Research Institutes, Biotechnology Companies, Pharmaceutical Companies
- Region: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Leading Companies: 3Brain AG, Alpha MED Scientific, Inc., Axion BioSystems, Inc., Blackrock Microsystems LLC, BMSEED LLC, FHC, Inc., Innovative Neurophysiology Inc., MaxWell Biosystems AG, MicroElectrodeDevices MED sàrl, Microprobes for Life Science, Multi Channel Systems MCS GmbH, NETRI, Neuralynx, Inc., NMI Technologie Transfer GmbH, Plexon Inc., Screen Holdings Co. Ltd, STEMCELL Technologies Canada Inc., Tucker-Davis Technologies, World Precision Instruments
Key Takeaways for Senior Decision-Makers
- Real-time, multi-site electrophysiological recording through in vitro micro electrode arrays is reshaping translational neuroscience and toxicology screening workflows.
- Recent advancements in substrate flexibility, microfabrication, and machine learning-based analytics have significantly broadened assay capabilities and data interpretation accuracy.
- Human-derived cell models are gaining traction, reducing reliance on animal testing and providing deeper insights while meeting evolving regulatory and ethical standards.
- End-users value modular and upgradeable instrumentation, open software architecture, and robust data management, responding to increased customization demands.
- Regional adoption varies, with established biopharma clusters prioritizing high-throughput utility, while emerging markets focus on foundational capabilities and collaborative infrastructure.
Tariff Impact on the In Vitro Micro Electrode Array Supply Chain
Newly imposed tariffs in key markets have led manufacturers to accelerate localization strategies and reassess sourcing channels. These adjustments aim to reduce input costs and supply chain risks, while distributors are optimizing inventory practices to counteract customs-related delays. The resulting shifts encourage innovation in value-added services and modular system design, enabling laboratories to maintain operational flexibility amid changing tariff landscapes.
Methodology & Data Sources
This report integrates primary interviews with leading electrophysiology experts, pharmaceutical R&D executives, and technical engineers, complemented by secondary analysis of peer-reviewed literature, corporate filings, and patent records. Data triangulation and expert validation ensure sound, actionable insights tailored for strategic decision-making.
Why This Report Matters
- Facilitates strategic planning by mapping technology trajectories, emerging segments, and competitive landscapes.
- Enables risk-mitigation through detailed assessment of regulatory and tariff influences on global supply chains and pricing.
- Equips leadership teams with segment-specific opportunities, supporting investment and partnership decisions across R&D and commercialization pipelines.
Conclusion
The in vitro micro electrode array market continues to evolve as an essential technology for predictive, scalable, and ethically aligned research. Senior leaders leveraging these insights can unlock new efficiencies and drive innovation within neuroscience and life science discovery.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this In vitro Micro Electrode Array market report include:- 3Brain AG
- Alpha MED Scientific, Inc.
- Axion BioSystems, Inc.
- Blackrock Microsystems LLC
- BMSEED LLC
- FHC, Inc.
- Innovative Neurophysiology Inc.
- MaxWell Biosystems AG
- MicroElectrodeDevices MED sàrl
- Microprobes for Life Science
- Multi Channel Systems MCS GmbH
- NETRI
- Neuralynx, Inc.
- NMI Technologie Transfer GmbH
- Plexon Inc.
- Screen Holdings Co. Ltd
- STEMCELL Technologies Canada Inc.
- Tucker-Davis Technologies
- World Precision Instruments
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 120.13 Million |
| Forecasted Market Value ( USD | $ 173.24 Million |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |