Speak directly to the analyst to clarify any post sales queries you may have.
Smart Orthopedic Implants Revolutionizing Patient Outcomes Through Integration of Advanced Sensor Technology and Personalized Therapeutic Solutions
Smart orthopedic implants represent a convergence of biomechanical engineering, data analytics, and patient-centered design that is redefining musculoskeletal care. By embedding miniaturized sensors and wireless connectivity, these next generation devices continuously monitor physiological parameters, enabling physicians to make real time adjustments to rehabilitation protocols. This integration of intelligent feedback loops enhances diagnostic precision and drives proactive interventions, reducing the incidence of post-operative complications.Beyond monitoring, intelligent implants facilitate personalized therapies through adaptive algorithms that calibrate device performance according to individual biomechanics. The ability to capture in vivo data on load distribution and range of motion offers unprecedented insight into patient recovery trajectories. Consequently, clinical teams can optimize rehabilitation regimens, accelerate mobility milestones, and improve long term outcomes.
As healthcare systems grapple with rising costs and a growing aging population, demand for solutions that deliver demonstrable value across the care continuum is intensifying. Smart orthopedic implants address this challenge by combining advanced materials science with digital health platforms. The resulting modalities promise to lower readmission rates, minimize revision surgeries, and elevate overall patient satisfaction.
Emerging Paradigm Shifts Transforming Orthopedic Treatment Modalities with Real Time Data Acquisition Connectivity and Adaptive Device Functionality
Orthopedic treatment modalities are undergoing fundamental transformation as the industry embraces connected devices, artificial intelligence, and predictive maintenance frameworks. The evolution from passive implants to intelligent systems reflects a broader shift toward data driven medicine, where continuous device telemetry informs clinical decisions. Real time monitoring enables early detection of mechanical stresses and biological responses, while predictive analytics anticipate complications before they manifest.Moreover, the integration of cloud based platforms and interoperability standards is accelerating collaborative care models. Surgeons, rehabilitation specialists, and data scientists are coalescing around unified digital ecosystems that aggregate longitudinal patient data. This holistic approach fosters evidence based protocols and iterative device enhancements informed by real world performance metrics.
Simultaneously, advances in additive manufacturing and materials engineering are converging to streamline customization processes. Intelligent design software leverages patient imaging to generate bespoke implant geometries, while connectivity modules ensure seamless post implantation communication. These innovations are not only reshaping surgical workflows but also cultivating new business models centered on outcome based reimbursement, setting the stage for a more agile, patient centric orthopedic landscape.
Comprehensive Evaluation of United States Tariff Impacts on Smart Orthopedic Device Supply Chains Manufacturing Costs and International Trade Dynamics
Recent tariff changes introduced by the United States trade authorities have introduced new variables into the strategic calculus for implant manufacturers and component suppliers. With increased duties on certain medical grade metals and polymeric materials, procurement teams are reevaluating their sourcing footprints to mitigate cost pressures. Supply chain diversification has become imperative as companies seek to balance domestic production incentives with access to high precision fabrication facilities abroad.The ripple effects of these policy adjustments extend to inventory management and capital investment decisions. Higher import levies have encouraged the repatriation of critical manufacturing activities, spurring capital expenditures in local machining centers and coating operations while also prompting renegotiations of long term supplier contracts. Concurrently, some firms are exploring free trade agreement zones and bonded warehousing structures to circumvent tariff escalations and preserve margin integrity.
Trade compliance functions are also intensifying scrutiny of classification codes and valuation methodologies. Companies with robust customs expertise are achieving greater clarity on eligibility for tariff exclusions and tariff rate quotas, thereby optimizing landed costs. This evolving regulatory landscape underscores the importance of agile supply chain strategies that can absorb fluctuations in trade policy without compromising product availability or clinical continuity.
Thorough Market Segmentation Exploring Performance Variations Across Implant Categories Biomaterial Selections Application Requirements and End User Settings
When dissecting the market by implant type, it becomes clear that stand alone solutions are diversifying beyond traditional orthotic supports to include wearable smart devices capable of real time biomechanical feedback. These devices are gaining traction among outpatient rehabilitation providers who value their non invasive nature and continuous monitoring capabilities. By contrast, surgically implanted components continue to focus on load bearing joint replacements, with hip implants benefiting from innovations in sensor enhanced stability while knee systems emphasize dynamic alignment feedback to optimize post operative gait.In the realm of biomaterials, metal based constructs maintain their prominence due to favorable strength to weight ratios and established clinical track records. Within this category, titanium alloys are increasingly preferred for their biocompatibility and corrosion resistance, whereas cobalt chrome retains relevance for high wear applications. Polymer based implants are growing in parallel, driven by advances in biodegradable formulations that gradually transfer stress to healing tissue, and non biodegradable polymers that offer tailored elasticity for soft tissue interface applications.
Applications in spinal and trauma care further delineate unique value propositions. Intelligent cervical and lumbar fixation devices are revolutionizing spinal stabilization through embedded motion sensors that aid surgical planning and post operative evaluation. Meanwhile, external and internal fixator systems are integrating wireless modules to monitor bone healing progress, offering trauma surgeons continuous insights into callus formation and alignment.
End user dynamics underscore a shift toward decentralized care. Ambulatory surgical centers are embracing compact, sensor enriched implant solutions that reduce hospital stays and drive efficiency. Hospitals remain pivotal for complex reconstructive procedures, leveraging comprehensive data analytics platforms to coordinate multi disciplinary teams around long term patient monitoring.
Regional Dynamics Shaping Smart Orthopedic Implant Adoption Patterns in the Americas Europe Middle East Africa and Asia Pacific Healthcare Ecosystems
In the Americas, a mature reimbursement landscape and widespread capabilities for advanced clinical research have fostered early adoption of smart orthopedic implants. North American health systems are collaborating with device developers on value based care trials, demonstrating reductions in revision rates and overall treatment costs. These evidentiary milestones are accelerating payer acceptance and expanding coverage pathways. Latin America is also emerging as a growth frontier, where increasing healthcare investment is catalyzing pilot deployments in key urban markets, often in partnership with regional orthopedic societies.Within Europe, Middle East and Africa, regulatory harmonization under the European Medical Device Regulation has elevated the bar for safety and performance documentation. As a result, leading European orthopedic centers are leveraging data analytics to refine patient selection criteria and refine post market surveillance. In the Middle East, sovereign wealth funded healthcare programs are procuring state of the art implant systems to build centers of excellence, while in Africa, targeted partnerships are advancing telehealth enabled rehabilitation protocols after initial device implantation.
The Asia Pacific region presents a dynamic mosaic of market conditions. Japan's advanced regulatory framework and aging demographic profile drive demand for sensor enriched implants, with local manufacturers pioneering miniaturized electronics integration. China is scaling smart implant production through government led innovation clusters, and India is witnessing a surge in low cost wearable orthotic solutions designed for rural outpatient clinics. Southeast Asian nations are selectively integrating these technologies within private hospital networks, leveraging strong medical tourism growth to attract specialized orthopedic care.
Strategic Analysis of Leading Industry Players Advancements Partnerships Innovation Pathways and Competitive Positioning in the Smart Orthopedic Implant Sector
Leading industry participants are investing heavily in sensor technology, digital therapeutics partnerships and cross sector alliances to solidify their market leadership. Several global orthopedic device manufacturers have established dedicated digital health divisions that collaborate with software firms to develop robust analytics dashboards. These initiatives are aimed at delivering closed loop feedback mechanisms that enable surgeons to adjust implant performance parameters remotely.Strategic acquisitions have further diversified product portfolios and accelerated time to market for intelligent solutions. By integrating early stage sensor developers and telemedicine platforms, established device companies have been able to offer end to end ecosystems spanning implantable hardware, mobile applications and clinician dashboards. Collaborative research agreements with academic medical centers underpin clinical validation studies that quantify improvements in rehabilitation speed and functional outcomes.
Competition is also intensifying among specialized smart implant disruptors. These agile innovators are focusing on modular components and scalable software architectures that can be retrofitted into existing implant designs. Their emphasis on interoperability standards and cloud native platforms is challenging traditional business models, prompting industry incumbents to evolve toward subscription based service offerings. Overall, the sector is characterized by a continuous cycle of technological convergence and strategic alignment as companies vie for differentiation.
Actionable Strategic Roadmap Empowering Industry Leaders to Capitalize on Smart Orthopedic Implant Innovations Optimize Operations and Drive Sustainable Growth
Industry leaders should prioritize the expansion of cross functional innovation labs that bring together engineers, clinicians and data scientists to co create next generation implantable systems. Early stage pilot programs conducted in real world clinical settings will accelerate technology validation and unlock reimbursement support. At the same time, establishing strategic partnerships with semiconductor and connectivity providers can ensure that implants remain compatible with evolving IoT ecosystems.Supply chain resilience must be reinforced through dual sourcing strategies and regional manufacturing hubs that mitigate the impact of trade policy fluctuations. Engaging in proactive regulatory dialogue with authorities will streamline approvals for novel materials and sensor integrations, while leveraging adaptive trial designs can expedite post market data collection. Furthermore, forging value based agreements with payers based on performance metrics can shift procurement toward long term outcomes rather than upfront device costs.
Investing in scalable data analytics platforms that integrate implant telemetry with electronic health records will empower care teams to derive actionable insights at scale. Leaders should also develop comprehensive training curricula to ensure that surgeons and rehabilitation specialists adopt data driven workflows. By aligning organizational structures with digital transformation objectives and emphasizing continuous improvement, companies can capture the full potential of smart orthopedic implants.
Robust Multimodal Research Design and Methodological Framework Underpinning the Comprehensive Examination of Smart Orthopedic Implant Market Dynamics
This research employs a dual approach that combines quantitative data aggregation with qualitative expert insights to produce a holistic market perspective. Primary interviews with orthopedic surgeons, rehabilitation specialists and device engineers were conducted to capture firsthand perspectives on clinical adoption barriers, technical requirements and patient experience enhancements. Secondary sources, including peer reviewed journals, regulatory filings and medical device registries, were systematically analyzed to corroborate emerging trends.Patent landscape assessments and clinical trial database reviews were utilized to identify breakthrough technologies and map competitive innovation pipelines. Supply chain analyses incorporated trade data and customs filings to assess the impact of recent tariff adjustments on production and distribution networks. Data triangulation was achieved through iterative validation sessions with an advisory board of subject matter experts, ensuring that findings reflect both macroeconomic influences and microscale device performance dynamics.
Statistical methods, including regression analysis and scenario modeling, were applied to historical implant usage patterns and reimbursement data. These insights were further contextualized through regional case studies that illustrate divergent regulatory pathways and healthcare infrastructure nuances. Throughout, methodological rigor was maintained via standardized data collection protocols and transparent documentation of assumptions, providing stakeholders with a credible foundation for strategic decision making.
Synthesis of Strategic Conclusions Illustrating Essential Takeaways from the Smart Orthopedic Implant Ecosystem Analysis and Future Industry Implications
The analysis of smart orthopedic implants underscores a paradigm shift toward devices that seamlessly integrate sensing capabilities with advanced materials and digital health platforms. Critical success factors include the ability to generate reliable in vivo data, navigate evolving regulatory environments and secure payer acceptance through demonstrated clinical value. The segmentation landscape reveals diverse applications across orthotic supports, joint replacements and fixation systems, each demanding tailored design and reimbursement strategies.Regional insights highlight that while mature markets are driving volume through value based care models, emerging economies are catalyzing adoption via public private partnerships and medical tourism initiatives. Leading players are balancing organic innovation with strategic acquisitions to build cohesive ecosystems that encompass hardware, software and service components. Supply chain realignment in response to tariff changes further illustrates the necessity of operational agility.
Looking ahead, cross sector collaboration among device manufacturers, technology firms and healthcare providers will accelerate the transition from discrete implants to holistic patient management solutions. Embracing data interoperability, adaptive trial designs and outcome oriented contracting will be essential for capturing the next wave of growth. Ultimately, stakeholders that align their innovation agendas with clinical imperatives and patient centric value propositions will be best positioned to thrive.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Type
- Stand-Alone Implants
- Orthotic Devices
- Wearable Smart Orthopedic Devices
- Surgical Implants
- Hip Implants
- Knee Implants
- Stand-Alone Implants
- Biomaterials Used
- Metal-Based Implants
- Cobalt Chrome Implants
- Titanium Implants
- Polymer-Based Implants
- Biodegradable Polymers
- Non-Biodegradable Polymers
- Metal-Based Implants
- Application
- Spinal Injuries
- Cervical Fixation Devices
- Lumbar Fixation Devices
- Trauma Injuries
- External Fixator Devices
- Internal Fixator Devices
- Spinal Injuries
- End-User
- Ambulatory Surgical Centers
- Hospitals
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Aesculap Implant Systems, LLC
- Aesculap Inc.
- Brainlab AG
- Cleveland Clinic Innovations
- Episurf Medical AB
- Exactech, Inc.
- Globus Medical, Inc.
- Implanet S.A.
- Johnson & Johnson
- Kinamed Inc.
- Materialise NV
- Medacta International SA
- Medtronic plc
- Merete GmbH
- MicroPort Scientific Corporation
- NuVasive, Inc.
- Onkos Surgical, Inc.
- OrthAlign, Inc.
- Orthofix Medical Inc.
- OrthoGrid Systems, Inc.
- Paragon 28, Inc.
- SeaSpine Holdings Corporation
- Stryker Corporation
- Think Surgical, Inc.
- Zimmer Biomet Holdings, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Smart Orthopedic Implants market report include:- Aesculap Implant Systems, LLC
- Aesculap Inc.
- Brainlab AG
- Cleveland Clinic Innovations
- Episurf Medical AB
- Exactech, Inc.
- Globus Medical, Inc.
- Implanet S.A.
- Johnson & Johnson
- Kinamed Inc.
- Materialise NV
- Medacta International SA
- Medtronic plc
- Merete GmbH
- MicroPort Scientific Corporation
- NuVasive, Inc.
- Onkos Surgical, Inc.
- OrthAlign, Inc.
- Orthofix Medical Inc.
- OrthoGrid Systems, Inc.
- Paragon 28, Inc.
- SeaSpine Holdings Corporation
- Stryker Corporation
- Think Surgical, Inc.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
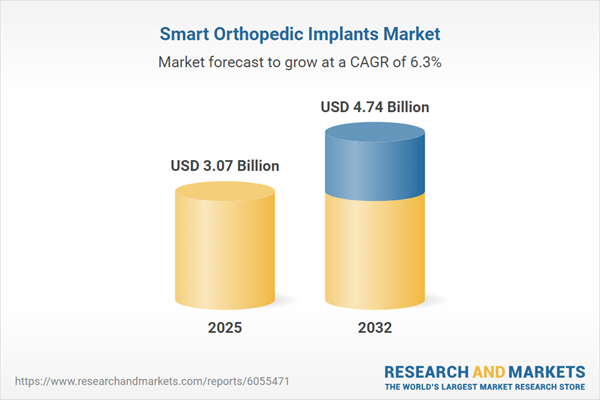
| Estimated Market Value ( USD | $ 3.07 Billion |
| Forecasted Market Value ( USD | $ 4.74 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |