Speak directly to the analyst to clarify any post sales queries you may have.
Dermal infusion devices are transforming non-invasive skin health solutions through advanced exfoliation, targeted serum delivery, and integrated treatment modalities. Senior decision-makers in medical aesthetics and beauty technology sectors can leverage these evolving technologies to enhance clinical offerings and address diverse patient expectations.
Market Snapshot: Dermal Infusion Devices Market Overview
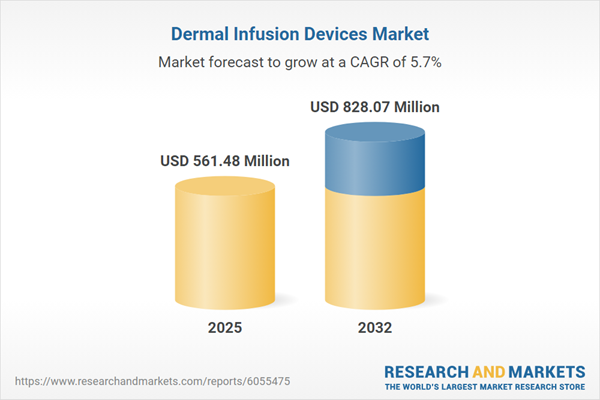
The Dermal Infusion Devices Market grew from USD 532.82 million in 2024 to USD 561.48 million in 2025. It is expected to continue growing at a CAGR of 5.66%, reaching USD 828.07 million by 2032.
Scope & Segmentation of the Dermal Infusion Devices Market
- Device Types: Portable, Tabletop
- Component Categories: Serum, Vacuum, Wand
- Material Types: Disposable, Reusable
- Technology Platforms: Hydradermabrasion, Infusion Roller, Ultrasound-Assisted Infusion, Vacuum Suction
- Power Sources: Battery Operated, Corded Electric
- Distribution Channels: Offline Sales, Online Sales, Brand Websites, E-commerce Platforms
- Applications: Acne Care (Pore Refining, Sebum Control), Body Contouring, Hair Rejuvenation, Skin Rejuvenation (Anti-aging, Hyperpigmentation, Texture Improvement)
- End-User Categories: Beauty Salons & Spas, Dermatology Clinics, Hospitals
- Regional Coverage: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Leading Companies: Including A.S.A.P., Inc., ADSS Group, Allergan PLC by AbbVie Inc., Altair Instruments, Inc., Aquavit Pharmaceuticals, Inc., Aspirus, Inc., Beauty Health Company, DCA Cosmetics, Derma Group LTD., DermaSweep, Inc., Genesis Biosystems, Inc, Hydrafacial LLC, Pollogen by Lumenis Be Ltd, Qure, Revance Therapeutics Inc., Venus Concept Inc., among others
Key Takeaways: Strategic Insights for Senior Decision-Makers
- Rapid adoption of dermal infusion platform technologies is bridging the gap between clinical and at-home skincare, enabling customizable protocols that appeal to a broad user base.
- Demand for personalized and application-specific serums is driving the integration of science-backed formulations with device compatibility, prompting manufacturers to expand product portfolios.
- Emergence of portable, battery-operated devices supports the shift to convenience in both professional and consumer channels, promoting access to high-performance skin treatments.
- Strengthening regulatory frameworks and quality management systems are impacting product launch strategies, requiring investment in compliance and device validation processes.
- Regional differentiation is pronounced, with Asia-Pacific setting innovation trends and Europe enforcing rigorous performance and safety standards, while the Americas leverage dual offline and online distribution models.
Tariff Impact on Dermal Infusion Device Supply Chains
- The 2025 United States tariffs are prompting industry participants to reassess international component sourcing for microprocessors and infusion wands.
- Organizations are optimizing local assembly, forming regional supplier agreements, and exploring modular device designs to reduce cost exposure and streamline compliance.
- Joint ventures with domestic firms and supply diversification efforts offer resilience against future policy changes and ensure workflow continuity despite evolving trade conditions.
Primary Keyword Focus: Dermal Infusion Devices Market
Senior decision-makers evaluating the Dermal Infusion Devices Market benefit from deep dives into segmentation, technology adoption, and cross-regional growth. Optimizing operations through strategic supply chain decisions, innovative partnerships, and regulatory engagement is crucial for sustainable advancement in this competitive sector. Secondary keywords addressed include 'clinical-grade technology' and 'hydradermabrasion trends'.
Methodology & Data Sources
This report synthesizes insights from primary interviews with industry stakeholders, secondary research spanning regulatory filings, trade publications, and clinical data reviews. Quantitative and qualitative analyses were triangulated for accuracy and actionability, with expert advisory input ensuring relevance for executive audiences.
Why This Report Matters
- Enables leaders to assess emerging opportunities driven by shifting demand for advanced, non-invasive skin health solutions.
- Provides clear segmentation analysis, technology coverage, and regional market trends to inform investment and go-to-market strategies.
- Guides organizations in structuring supply chains and partnerships to remain resilient amid trade policy shifts.
Conclusion
The dermal infusion devices market is evolving quickly in both offering and application, setting new standards in personalized and non-invasive care. Senior decision-makers can derive strategic value by leveraging this report’s actionable insights for market positioning and operational resilience.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Dermal Infusion Devices market report include:- A.S.A.P., Inc.
- ADSS Group
- Allergan PLC by AbbVie Inc.
- Altair Instruments, Inc.
- Aquavit Pharmaceuticals, Inc.
- Aspirus, Inc.
- Beauty Health Company
- DCA Cosmetics
- Derma Group LTD.
- DermaSweep, Inc.
- Design Catapult Manufacturing
- Genesis Biosystems, Inc
- Glov Beauty
- Hydrafacial LLC
- Kindly Group
- LUVO
- Pollogen by Lumenis Be Ltd
- ProSkin
- Qure
- Revance Therapeutics Inc.
- Skeyndor
- Skin Gym USA
- Skin Science Solutions
- Skinnovation
- Sofi Cosmetic Group
- SuSkin
- Venus Concept Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 561.48 Million |
| Forecasted Market Value ( USD | $ 828.07 Million |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |