Speak directly to the analyst to clarify any post sales queries you may have.
The Biolayer Interferometry Instruments Market is evolving rapidly, presenting organizations with new methods for analyzing molecular interactions and advancing R&D objectives. Adoption of advanced interferometry systems promises tangible advantages for leaders optimizing workflows in research, diagnostics, and pharmaceutical development.
Market Snapshot: Growth Trajectory of the Biolayer Interferometry Instruments Market
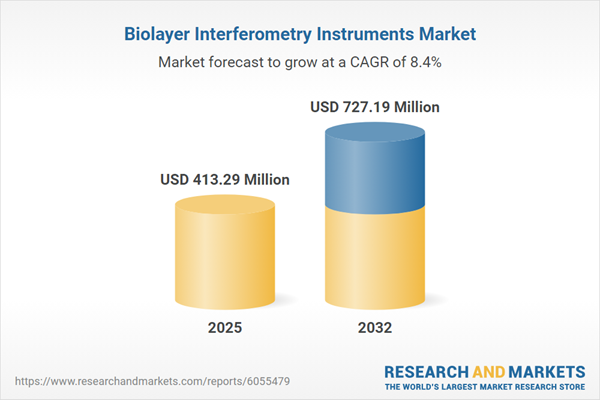
The biolayer interferometry instruments market grew from USD 382.74 million in 2024 to USD 413.29 million in 2025. It is forecast to expand at a CAGR of 8.35%, reaching USD 727.19 million by 2032. This growth reflects accelerating demand for real-time, label-free biomolecular interaction analysis across diverse applications and regions. The market continues to benefit from industry investments in automation, data analytics, and streamlined laboratory operations, positioning biolayer interferometry as an important tool for organizations responding to heightened quality demands and regulatory complexity.
Scope & Segmentation of the Biolayer Interferometry Instruments Market
- Component: Consumables (biosensors and kits), instruments, and solutions including data analysis software and maintenance services.
- Application: Biotechnology research, diagnostics, drug discovery, and quality control.
- End User: Academic institutions, biotechnology companies, contract research organizations, pharmaceutical industries.
- Regions: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East, and Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Technologies and Trends: Multiplexed detection, AI-driven data processing, modular instrument designs, and cloud-based software ecosystems.
Key Takeaways for Market Decision-Makers
- Biolayer interferometry enables real-time biomolecular analysis without the need for labeling, supporting high-throughput automation for laboratories.
- Platform usability and integration with advanced analytics software contribute to reduced complexity in workflows and improved accuracy in data interpretation.
- Modular designs and customizable configurations address evolving research objectives and operational budgets, while supporting scalability in instrument deployment.
- Collaboration among instrumentation developers, software providers, and service organizations drives the market toward comprehensive, bundled solutions tailored to diverse user needs.
- Regional expansion is facilitated by government incentives, localized service support, and unique regulatory environments, highlighting the relevance of targeted product strategies for global adoption.
Tariff Impact and Supply Chain Adaptations
Recent United States tariff policies have introduced new layers of complexity to global supply chains for biolayer interferometry technology. Increased duties on imported optical components and raw materials have led manufacturers to reconsider sourcing options and accelerate efforts to localize production. This shift encourages the establishment of regional hubs and enhanced inventory management, with organizations diversifying logistics partnerships for greater resilience. The sector’s adaptability is evident in new collaborations that enable local manufacturing and improved regulatory alignment, safeguarding operational continuity amid shifting trade dynamics.
Methodology & Data Sources
The market insights are grounded in a multi-stage methodology integrating primary interviews with industry executives and end users, analysis of peer-reviewed publications, regulatory filings, and quantitative modeling. Structured input from R&D leadership, procurement managers, and regulatory specialists ensures the findings address procurement practices and adoption challenges. Robust data triangulation and scenario analysis validate the outcomes and strategic relevance for decision-makers.
Why This Report Matters
- Delivers actionable market segmentation that supports targeted investment in technology, workflow optimization, and service innovation.
- Equips senior leaders with insight into regional and global dynamics, guiding informed responses to regulatory, supply chain, and competitive developments.
- Presents a credible foundation for benchmarking partner and vendor strategies, enabling agile adaptation to market and policy shifts.
Conclusion
The biolayer interferometry instruments market presents robust opportunities as organizations pursue greater efficiency, data quality, and regulatory compliance. Leaders who prioritize integration, flexibility, and regional alignment will be well-positioned to achieve sustained growth and competitive advantage.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Biolayer Interferometry Instruments market report include:- Agilent Technologies, Inc
- Amerigo Scientific
- Attana AB
- Bio-Rad Laboratories
- Bionavis Ltd
- Biosensors International Group, Ltd.
- Biosensors Japan Co., Ltd.
- BMG LABTECH
- Bruker Corporation
- Danaher Corporation
- Dynamic Biosensors GmbH
- Gator Bio
- GenScript Biotech Corporation
- Hangzhou Freqcontrol Electronic Technology Ltd.
- Horiba Ltd.
- IST AG
- Malvern Panalytical Ltd by Spectris PLC
- NanoTemper Technologies GmbH
- Nicoya Lifesciences
- Sartorius AG
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 413.29 Million |
| Forecasted Market Value ( USD | $ 727.19 Million |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |