Speak directly to the analyst to clarify any post sales queries you may have.
The facial palsy market is undergoing significant transformation, driven by advancements in therapeutic modalities, evolving healthcare policies, and substantial shifts in patient demographics. Leading stakeholders across the healthcare and rehabilitation sectors face complex decisions as new interventions, technologies, and regional market forces shape the competitive landscape.
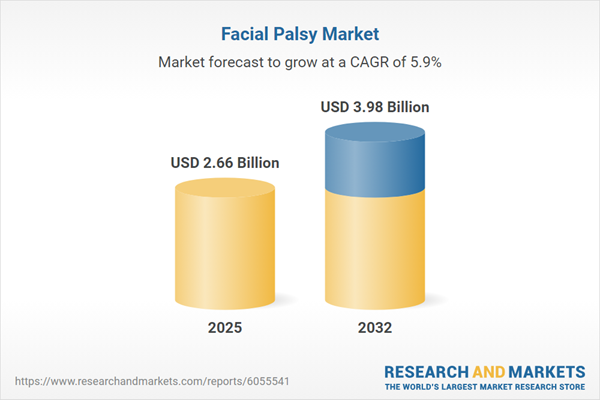
Market Snapshot: Facial Palsy Market Size and Growth
The global facial palsy market grew from USD 2.52 billion in 2024 to USD 2.66 billion in 2025. With a forecasted CAGR of 5.85%, the market is projected to reach USD 3.98 billion by 2032. This growth reflects strong momentum across both existing and emerging treatment segments, further substantiated by surging demand for improved patient outcomes and personalized care.
Scope & Segmentation
- Type: Central facial palsy (stroke-induced, traumatic brain injury), Peripheral facial palsy (Bell's palsy, Lyme disease-induced, Ramsay Hunt syndrome)
- Treatment Type: Non-pharmacological therapies (acupuncture, electrical stimulation, physiotherapy), Pharmacological therapies (analgesics, antiviral medications, corticosteroids), Surgical interventions (muscle transfers, nerve grafting)
- Therapy Duration: 3 to 6 months, Less than 3 months, More than 6 months
- Age Group: Adult, Geriatric, Pediatric
- Severity of Condition: Mild, Moderate, Severe
- End-User: Ambulatory surgical centers, Clinics (neurological, rehabilitation), Homecare settings, Hospitals (general, specialty)
- Geographical Coverage: Americas (North America, Latin America), Europe, Middle East & Africa, Asia-Pacific. Markets such as the United States, Canada, China, India, United Kingdom, Germany, and Australia are addressed, reflecting critical hubs for facial palsy intervention and innovation.
- Leading Companies: AbbVie Inc., Astellas Pharma Inc., Bayer AG, Bristol-Myers Squibb Company, Cepheid by Danaher Corporation, Coloplast A/S, F. Hoffmann-La Roche AG, GlaxoSmithKline plc, Integra LifeSciences Holdings Corporation, Ipsen Biopharmaceuticals, Inc., Johnson & Johnson Services, Inc., Kenvue Brands LLC, Mallinckrodt plc, Merz Pharmaceuticals, LLC, Novartis AG, Reckitt Benckiser Group PLC, Revance Therapeutics, Inc. by Crown Laboratories, Inc., Sanofi SA, Stryker Corporation, Sucampo Pharmaceuticals, Teva Pharmaceutical Industries Ltd.
Key Takeaways: Strategic Insights for the Facial Palsy Market
- Therapeutic innovation is driven by multimodal care strategies that combine digital health, nonpharmacological therapies, and refined microsurgical techniques, enhancing patient-centric outcomes.
- Industry stakeholders are prioritizing agile decision-making and cross-sector collaborations, leveraging both academic partnerships and medtech synergies to expedite research and market entry.
- Healthcare providers increasingly incorporate telehealth and digital rehabilitation, meeting the diverse needs of pediatric, adult, and geriatric populations through adaptable service models.
- Market segmentation enables more precise resource allocation and targeting, with age group, therapy duration, and treatment setting influencing strategy development and patient engagement approaches.
- Regional innovation ecosystems, particularly in Asia-Pacific and the Americas, benefit from expanding hospital infrastructure, technology transfer, and public-private initiatives, promoting access and cost-efficiency.
Tariff Impact: Evolving Supply Chains and Cost Strategies
The introduction of revised United States tariffs in 2025 has catalyzed notable shifts in supply chain resilience, especially for imported medical devices, reagents, and pharmaceutical ingredients. Increased duties on neuromodulation equipment and critical compounds have prompted manufacturers to localize production, renegotiate sourcing agreements, and form alliances with domestic OEMs. These measures have introduced additional procurement challenges and accelerated adaptation strategies, particularly for sectors reliant on advanced surgical instrumentation and specialty pharmaceuticals. Healthcare providers are responding with renewed focus on nonpharmacological interventions where possible, optimizing both treatment outcomes and cost structures.
Methodology & Data Sources
This facial palsy market analysis employs interviews with key opinion leaders, clinicians, rehabilitation specialists, and payers across priority regions. Extensive desk research draws from clinical trials, regulatory filings, peer-reviewed journals, and proprietary device approval databases. The approach integrates quantitative modeling and scenario planning to evaluate tariff impacts, demographic shifts, and therapy adoption patterns, supplemented by qualitative insights from stakeholder interviews and thematic coding.
Why This Report Matters
- Equips executives and investors with actionable intelligence for strategic planning, portfolio optimization, and partnership development across the facial palsy care continuum.
- Reveals critical trends around digital health, tariff impacts, and patient segmentation, facilitating data-driven decisions and targeted innovation investment.
- Enables supply chain leaders, clinical teams, and policy strategists to respond effectively to regulatory, technological, and market pressures in a complex therapeutic landscape.
Conclusion
The facial palsy market presents a dynamic environment shaped by technological advancement, adaptive supply strategies, and deepening patient segmentation. Decision-makers who adopt a flexible, evidence-driven approach will be best positioned to lead in this evolving sector.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Facial Palsy market report include:- AbbVie Inc.
- Astellas Pharma Inc.
- Bayer AG
- Bristol-Myers Squibb Company
- Cepheid by Danaher Corporation
- Coloplast A/S
- F. Hoffmann-La Roche AG
- GlaxoSmithKline plc
- Integra LifeSciences Holdings Corporation
- Ipsen Biopharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Kenvue Brands LLC
- Mallinckrodt plc
- Merz Pharmaceuticals, LLC
- Novartis AG
- Reckitt Benckiser Group PLC
- Revance Therapeutics, Inc. by Crown Laboratories, Inc.
- Sanofi SA
- Stryker Corporation
- Sucampo Pharmaceuticals
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.66 Billion |
| Forecasted Market Value ( USD | $ 3.98 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |