Speak directly to the analyst to clarify any post sales queries you may have.
Building a Comprehensive Foundation for Understanding the Complex Dynamics of Cattle and Porcine Reproductive Health Challenges
The agricultural sector faces persistent challenges stemming from reproductive diseases that undermine productivity in bovine and porcine populations. Unraveling the intricate etiology of ailments such as bovine viral diarrhea and porcine reproductive and respiratory syndrome is crucial to mitigating losses in dairy and beef cattle as well as in breeding swine. Understanding the pathophysiology of these conditions, from infection vectors to transmission dynamics, provides the foundation for targeted interventions that sustain animal health and safeguard economic viability.This executive summary synthesizes critical developments across diagnostic breakthroughs, therapeutic innovations, and regulatory shifts that are reshaping disease management strategies. As veterinary professionals and industry stakeholders navigate evolving disease prevalence, this analysis offers a structured view of prevailing trends and identifies key inflection points. By establishing a comprehensive context for the current landscape, this introduction sets the stage for deeper insights into market segmentation, regional variations, and strategic imperatives that drive decision making in both cattle and swine reproductive health management.
Moreover, the collaborative efforts of epidemiologists, veterinarians, and molecular biologists are forging a more resilient framework for disease surveillance and control. Integral to these efforts is the integration of advanced diagnostics and data analytics, which together enable real-time monitoring and rapid response. This introduction therefore not only frames the current state of reproductive health challenges but also highlights the multidisciplinary momentum propelling the sector toward more robust, sustainable outcomes.
Driving Evolution in Bovine and Porcine Reproductive Health Amid Shifts in Technology and Policy
A convergence of cutting-edge diagnostic techniques and evolving policy frameworks is revolutionizing the way reproductive diseases are identified and managed in cattle and swine. Innovations in molecular methods, including rapid PCR assays and loop-mediated isothermal amplification, have elevated the precision of early detection, while advances in serological tests such as enzyme-linked immunosorbent assays and serum neutralization tests are providing deeper insights into herd immunity profiles. Concurrently, ultrasonography is gaining traction as an indispensable tool for real-time reproductive assessment, enabling practitioners to detect abnormalities with unprecedented accuracy.In parallel, the rise of precision livestock farming is creating new pathways for continuous health monitoring. Sensor technologies, automated data capture, and predictive analytics are now being harnessed to anticipate disease outbreaks before clinical symptoms emerge. These technological shifts are complemented by policy initiatives aimed at reducing antimicrobial use and promoting animal welfare, which are reshaping treatment protocols and driving demand for vaccine innovations and alternative therapies.
As these developments take hold, stakeholders are recalibrating their approaches to research, development, and field implementation. Emphasis is shifting from reactive disease management toward proactive, data-driven strategies that prioritize prevention and resilience. This transformative momentum signifies a fundamental shift in reproductive health paradigms, setting the stage for more sustainable and effective interventions across the cattle and porcine segments.
Assessing the Far-Reaching Consequences of 2025 United States Tariff Adjustments on Veterinary Disease Management Resources
The implementation of new tariff measures in 2025 has introduced significant headwinds into the supply chains underpinning veterinary diagnostics and therapeutics. Import duties on diagnostic reagents, molecular testing kits, and specialized imaging equipment have elevated procurement costs, compelling industry participants to reassess sourcing strategies and inventory management practices. These financial pressures have reverberated across laboratories and field clinics, prompting a shift toward domestic manufacturing capabilities and local partnerships aimed at mitigating exposure to international trade fluctuations.Moreover, tariffs on pharmaceutical imports, encompassing vaccines and antiviral agents, have disrupted established distribution networks. This has led to extended lead times and variable pricing, prompting veterinarians and farm operators to explore alternative treatment modalities such as herbal formulations and complementary homoeopathic interventions. In response, manufacturers are exploring regional production hubs and forging strategic alliances to secure supply continuity and optimize cost structures.
Despite these challenges, the tariff environment has also catalyzed innovation. Entities are accelerating investments in point-of-care diagnostic platforms and modular vaccine production technologies that can be scaled rapidly in response to localized demand. By leveraging these adaptive strategies, stakeholders are not only navigating tariff-induced constraints but also laying the groundwork for a more resilient and agile industry ecosystem.
Illuminating Market Depth through Multifaceted Segmentation Insights Covering Type, Animal, Diagnostics, Treatments, and End Users
In examining the market through the prism of disease type, it becomes clear that conditions such as bovine viral diarrhea and infectious bovine rhinotracheitis continue to dominate concerns in cattle, while porcine reproductive and respiratory syndrome and swine dysentery remain top priorities in swine. Brucellosis, leptospirosis, and neosporosis persist as chronic threats across both bovine and porcine populations, calling for integrated diagnostic and preventive measures. Furthermore, less prevalent but economically significant diseases such as trichomoniasis and campylobacteriosis in cattle, as well as porcine parvovirus in swine, require specialized attention within broader herd health strategies.When viewed by animal type, the differentiation between beef and dairy cattle introduces distinct management imperatives, while the unique life stages of swine-spanning breeding sows to finishing and nursery pigs-demand tailored diagnostic protocols and treatment regimens. This animal-type framework underscores the necessity for customizable solutions that align with production goals and welfare standards.
Delving into diagnostic methodologies, molecular methods like PCR and loop-mediated isothermal amplification are paired with culturing techniques for bacterial and viral isolation, creating comprehensive testing algorithms. Diagnostic imaging, including ultrasonography and X-ray imaging, complements serological assays such as ELISA and AGID to deliver holistic health assessments. On the treatment front, vaccine portfolios span from inactivated to live attenuated formulations, supported by pharmaceutical interventions comprising antibiotics, antiparasitics, and antivirals, alongside alternative therapies rooted in herbal and homoeopathic principles.
Finally, the end-user segmentation highlights the pivotal roles of farms, veterinary clinics, and research institutions, each operating within distinct regulatory and operational frameworks. Farms serve as the primary site of disease manifestation and control, clinics function as diagnostic and treatment hubs, and research institutions drive innovation and validation of emerging technologies, forming an interconnected ecosystem that advances reproductive health management.
Decoding Regional Variability in Reproductive Disease Control across the Americas, Europe-Middle East-Africa, and Asia-Pacific Markets
In the Americas, an established livestock industry infrastructure supports widespread adoption of advanced diagnostics and therapeutics, with regulatory frameworks that encourage vaccine innovation and precision farming practices. The region's robust research institutions and extensive farm networks facilitate pilot programs that integrate molecular assays, imaging technologies, and data analytics into standard herd health protocols. This dynamic fosters a culture of continuous improvement and positions North and South American stakeholders at the forefront of disease surveillance and response.Europe, Middle East & Africa present a mosaic of regulatory environments and economic conditions, ranging from highly regulated European Union markets with stringent animal welfare mandates to emerging economies where access to cutting-edge diagnostics may be more constrained. Nonetheless, this region benefits from collaborative research consortia and public-private partnerships that drive harmonization of standards and the dissemination of best practices. The result is a gradual alignment toward integrated surveillance systems and cost-effective intervention strategies.
In Asia-Pacific, rapid growth in livestock production is met with rising investments in local manufacturing of veterinary products and the deployment of mobile diagnostics tailored to remote locations. Countries in this region are forging policy initiatives that balance disease control with sustainable agricultural development, incentivizing the adoption of point-of-care tests and modular vaccine platforms. These efforts not only address regional biosecurity concerns but also create opportunities for scaling solutions across diverse farming systems.
Profiling Leading Industry Participants Driving Innovation in Therapeutics, Diagnostics, and Strategic Collaborations
Major global players continue to expand their footprints through strategic alliances, research collaborations, and targeted acquisitions that bolster their product pipelines. Leading pharmaceutical entities are investing heavily in next-generation vaccine platforms, including recombinant and adjuvanted formulations designed to elicit robust, long-lasting immunity against key pathogens. This pipeline diversification reflects a strategic pivot from traditional vaccines toward precision immunotherapies that address emerging viral and bacterial strains.Diagnostic manufacturers are similarly intensifying efforts to integrate digital platforms with laboratory workflows, enabling seamless data capture and real-time disease monitoring. Partnerships between tech startups and established laboratories are accelerating the commercialization of portable PCR devices and automated imaging solutions, thus democratizing access to high-fidelity testing in field settings.
Regional specialists and contract research organizations are leveraging their localized expertise to support product registration and field trials, navigating diverse regulatory landscapes to expedite market entry. By combining global R&D capacity with regional distribution networks, these stakeholders are advancing a more collaborative and agile ecosystem that delivers tailored solutions to meet evolving needs in cattle and porcine reproductive health management.
Strategic Imperatives for Strengthening Supply Chains, Digital Integration, and Collaborative Innovation in Reproductive Health Management
Industry leaders should prioritize investment in modular manufacturing capabilities that can pivot rapidly between vaccine types and generics production, thereby reducing dependency on volatile international supply chains. By establishing decentralized production hubs, organizations can mitigate tariff risks and reinforce supply continuity, ensuring uninterrupted access to critical prophylactic and therapeutic solutions.Embracing integrated digital platforms for herd health management will enable real-time analytics and predictive modeling, empowering stakeholders to anticipate outbreaks and deploy interventions with precision. The development of user-centric mobile applications and cloud-based dashboards can streamline decision making across farms, clinics, and research institutions.
Cross-sector collaboration, including partnerships with academic research centers and technology startups, will accelerate the translation of emerging molecular diagnostics and advanced imaging into commercialized offerings. Co-innovation models can distribute development costs and align incentives toward shared outcomes, fostering a culture of continuous improvement.
Finally, adopting a One Health perspective that underscores the interconnectedness of animal, human, and environmental health will drive holistic strategies. Integrating biosecurity protocols with sustainable farming practices and community engagement will not only improve disease outcomes but also strengthen stakeholder trust and regulatory alignment.
Outlining a Rigorous Multimodal Research Framework Integrating Secondary Data Analysis and Expert Interviews for Veterinary Health Insights
This research methodology combines comprehensive secondary research with targeted primary engagements to ensure the robustness of findings. Initially, extensive literature reviews of peer-reviewed journals, government publications, and industry white papers provided foundational insights into disease prevalence, diagnostic advancements, and treatment modalities. These sources were critically evaluated to identify prevailing trends and emerging technology landscapes.Subsequently, structured interviews with subject matter experts-including veterinary epidemiologists, molecular biologists, and livestock management professionals-offered real-world perspectives on market challenges and innovation trajectories. Discussions focused on barriers to adoption, regulatory considerations, and the commercial viability of novel diagnostic and therapeutic solutions. Insights from these interviews were triangulated with secondary data to validate emerging themes and ensure consistency.
Quantitative data analysis techniques were employed to interpret regional and segment-specific variables, with cross-validation against industry benchmarks and historical performance metrics. The methodological framework prioritized transparency and reproducibility, with each analytical step documented to facilitate peer review and stakeholder scrutiny. This rigorous approach underpins the credibility of the strategic insights presented throughout this executive summary.
Synthesizing Segmentation, Regional Dynamics, and Strategic Recommendations to Illuminate the Path Forward for Reproductive Health Management
This executive summary has traced the evolving landscape of cattle and porcine reproductive health, highlighting the transformative impact of advanced diagnostics, policy shifts, and supply chain realignments. By examining disease prevalence through multifaceted segmentation lenses, readers gain a nuanced understanding of how pathogen dynamics interact with production systems, diagnostic methodologies, and treatment protocols.Regional analysis underscores the diverse trajectories of livestock industries across the Americas, Europe-Middle East-Africa, and Asia-Pacific, reflecting variations in regulatory environments, infrastructure maturity, and innovation adoption. Concurrently, profiles of leading companies reveal a collaborative ecosystem that is increasingly leveraging digital integration and strategic alliances to accelerate product development and market penetration.
Looking ahead, actionable recommendations focused on modular manufacturing, digital platform deployment, cross-sector collaboration, and a One Health approach provide a roadmap for industry leaders to navigate tariff pressures and emerging disease threats. Grounded in a rigorous research methodology, these insights equip decision-makers with the strategic clarity needed to drive sustainable improvements in reproductive health management.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Disease Type
- Bovine Viral Diarrhea (BVD)
- Brucellosis
- Campylobacteriosis
- Infectious Bovine Rhinotracheitis (IBR)
- Leptospirosis
- Neosporosis
- Porcine Parvovirus (PPV)
- Porcine Reproductive and Respiratory Syndrome (PRRS)
- Swine Dysentery
- Trichomoniasis
- Animal Type
- Cattle
- Beef Cattle
- Dairy Cattle
- Swine
- Breeding Swine
- Finishing Pigs
- Nursery Pigs
- Cattle
- Diagnosis Type
- Culturing Techniques
- Bacterial Culture
- Viral Isolation
- Diagnostic Imaging
- Ultrasonography
- X-Ray Imaging
- Molecular Methods
- LAMP Assays
- PCR
- Serological Tests
- AGID
- ELISA
- SNT
- Culturing Techniques
- Treatment Type
- Alternative Therapies
- Herbal Treatments
- Homoeopathy
- Pharmaceuticals
- Antibiotics
- Antiparasitics
- Antivirals
- Surgical Interventions
- Corrective Surgeries
- Prophylactic Surgeries
- Vaccinations
- Inactivated Vaccines
- Live Attenuated Vaccines
- Alternative Therapies
- End-User
- Farms
- Research Institutions
- Veterinary Clinics
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Anicon Labor GmbH
- Biogenesis Bago SA
- Biomin Holding GmbH
- Boehringer Ingelheim Animal Health
- CEVA Santé Animale
- Dechra Pharmaceuticals PLC
- Eco Animal Health Group plc
- Elanco Animal Health Incorporated
- Hipra
- Huvepharma EOOD
- Huvepharma Inc.
- IDEXX Laboratories, Inc.
- Innovacyn, Inc.
- Kaneka Corporation (via subsidiary)
- Medovent GmbH
- Merck Animal Health
- MSD Animal Health
- Neogen Corporation
- Norbrook Laboratories Ltd.
- Vetoquinol Global
- Virbac S.A.
- Zoetis Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Cattle & Porcine/Swine Reproductive Diseases market report include:- Anicon Labor GmbH
- Biogenesis Bago SA
- Biomin Holding GmbH
- Boehringer Ingelheim Animal Health
- CEVA Santé Animale
- Dechra Pharmaceuticals PLC
- Eco Animal Health Group plc
- Elanco Animal Health Incorporated
- Hipra
- Huvepharma EOOD
- Huvepharma Inc.
- IDEXX Laboratories, Inc.
- Innovacyn, Inc.
- Kaneka Corporation (via subsidiary)
- Medovent GmbH
- Merck Animal Health
- MSD Animal Health
- Neogen Corporation
- Norbrook Laboratories Ltd.
- Vetoquinol Global
- Virbac S.A.
- Zoetis Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
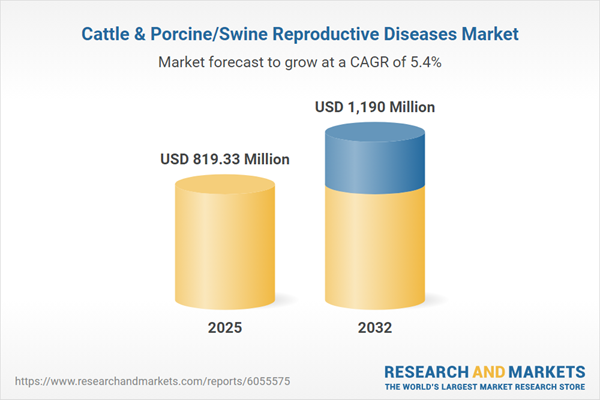
| Estimated Market Value ( USD | $ 819.33 Million |
| Forecasted Market Value ( USD | $ 1190 Million |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |