Speak directly to the analyst to clarify any post sales queries you may have.
The rare diseases treatment market is entering a pivotal phase marked by advanced therapeutic innovation, multi-stakeholder collaboration, and fundamental changes to research and development processes. As global demand for effective rare disease therapies continues to expand, industry leaders are navigating a landscape shaped by emerging technologies, evolving regulatory frameworks, and new patient engagement models.
Market Snapshot: Rare Diseases Treatment Market Outlook
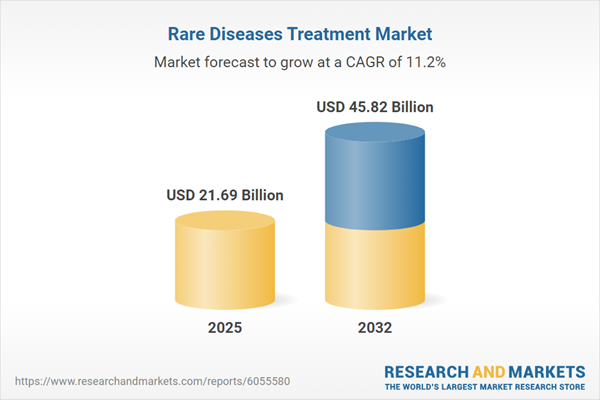
The rare diseases treatment market grew from USD 19.62 billion in 2024 to USD 21.69 billion in 2025 and is forecast to reach USD 45.82 billion by 2032—reflecting an impressive CAGR of 11.17%. This trajectory is underpinned by breakthroughs in gene therapies, enhanced diagnostic tools, and robust investment from both established pharmaceutical companies and agile biotechnology firms. Accelerated regulatory approvals and incentives for orphan drugs are influencing the market’s rapid evolution, while patient advocacy and digital health technologies are increasingly driving strategic decisions. These factors collectively reinforce the sector’s position as a high-growth and dynamic domain within global healthcare.
Scope & Segmentation
This comprehensive research covers a wide spectrum of market segments, addressing the full value chain and major geographic regions:
- Drug Type: Biological Drugs, Non-Biological Drugs
- Therapeutic Area: Cancer, Cardiovascular Conditions, Endocrine Disorders, Hematologic Disorders, Infectious Diseases, Metabolic Disorders, Musculoskeletal Conditions, Neurological Conditions
- Route of Administration: Injectable, Oral
- Distribution Channel: Hospital Pharmacy, Online Pharmacy, Specialty Pharmacy
- End User: Biotechnology and Pharmaceutical Companies, Government and Regulatory Bodies, Hospitals and Specialty Clinics, Patient Advocacy Groups and Nonprofits, Physician Practices and Outpatient Centers, Research and Academic Institutions
- Regional Coverage: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Leading Companies Profiled: AbbVie Inc., ACADIA Pharmaceuticals Inc., Alnylam Pharmaceuticals, Amgen Inc, AstraZeneca PLC, Baxter International, Bayer AG, Biogen Inc., BioMarin Pharmaceutical, Bristol-Myers Squibb Company, Editas Medicine, Eli Lilly and Company, F. Hoffmann La Roche Ltd, GSK plc, Johnson & Johnson Services, Merck KGaA, Novartis AG, Novo Nordisk A/S, Pfizer, Regenxbio Inc., Sanofi SA, Takeda Pharmaceutical Company Limited, United Therapeutics Corporation, Vertex Pharmaceutical, Inc.
Key Takeaways for Senior Decision-Makers
- Scientific advances, including gene editing and cell therapy, are driving transformational change from initial research through to clinical implementation.
- Adaptive regulatory approaches such as expedited pathways and real-world evidence requirements are enhancing the pace of drug development and approval.
- Patient-centered innovation models are embedding real-world insights into pipeline decisions, driving both improved outcomes and market alignment.
- Segmentation strategies, particularly across therapeutic domains and distribution channels, are critical for targeting high-potential drug modalities and optimizing patient access.
- Strategic partnerships—spanning pharmaceutical firms, biotech innovators, academia, and advocacy organizations—are emerging as key levers for accelerating discovery and commercialization.
- Regional customization remains essential, as reimbursement conditions and healthcare infrastructure vary substantially across major territories.
Tariff Impact: Navigating a Changing Supply Chain Environment
Forthcoming trade tariffs effective from 2025 are set to reshape the rare diseases treatment market’s supply chain and cost structures. Companies reliant on imported inputs are actively reassessing their sourcing models, with nearshoring and supply base diversification gaining traction to limit tariff exposure and logistical risk. This regulatory environment has spurred increased collaboration across procurement, manufacturing, and logistics stakeholders, driving greater supply chain resilience and adaptive scenario planning. Transparent dialogue with authorities is also becoming vital to address classification and access issues for critical therapies.
Methodology & Data Sources
This research integrates expert interviews, secondary literature review, and quantitative data analysis to capture an accurate and nuanced snapshot of the rare diseases treatment market. Clinical investigators, regulatory stakeholders, and advocacy leaders contributed insights through qualitative discussions, complemented by robust desk research and expert peer review. Quantitative analysis was performed to assess pipelines, resource allocation, and engagement models, ensuring rigor and comprehensive coverage across findings.
Why This Report Matters
- Drive informed, high-impact decisions with detailed segmentation, leading company profiles, and analysis of regulatory and supply chain dynamics.
- Anticipate and manage risks arising from regulatory shifts, evolving patient expectations, and upcoming trade policies that could impact business continuity and growth.
- Develop forward-looking strategies that combine adaptive market access, technological innovation, and regional customization for long-term relevance.
Conclusion
With complex challenges and unprecedented opportunities emerging, stakeholders in rare diseases therapeutics can accelerate innovation and enhance patient outcomes by leveraging integrated insights, adaptive supply chains, and collaborative partnerships.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Rare Diseases Treatment market report include:- AbbVie Inc.
- ACADIA Pharmaceuticals Inc.
- Alnylam Pharmaceuticals, Inc.
- Amgen Inc
- AstraZeneca PLC
- Baxter International
- Bayer AG
- Biogen Inc.
- BioMarin Pharmaceutical Inc.
- Bristol-Myers Squibb Company
- Editas Medicine, Inc.
- Eli Lilly and Company
- F. Hoffmann La Roche Ltd
- GSK plc
- Johnson & Johnson Services, Inc.
- Merck KGaA
- Novartis AG
- Novo Nordisk A/S
- Pfizer, Inc.
- Regenxbio Inc.
- Sanofi SA
- Takeda Pharmaceutical Company Limited.
- United Therapeutics Corporation
- Vertex Pharmaceutical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 21.69 Billion |
| Forecasted Market Value ( USD | $ 45.82 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |