Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive Overview of IgA Nephropathy Highlighting Pathophysiology Clinical Heterogeneity and the Urgent Demand for Innovative Therapeutic Approaches
IgA nephropathy, often referred to as Berger's disease, stands as the most common primary glomerulonephritis worldwide. It is characterized by the deposition of immunoglobulin A immune complexes within the mesangial region of the renal glomeruli. Since its first description in the mid-20th century, the disorder has presented clinicians and researchers with a formidable challenge due to its variable clinical manifestations and unpredictable progression. Geographically, prevalence is particularly high in East Asia but remains a significant cause of chronic kidney disease across Europe and the Americas. The increasing recognition of this disorder has been driven by improvements in biopsy techniques and novel imaging modalities, which have enhanced diagnostic accuracy and fostered a deeper understanding of its pathophysiology.At the mechanistic level, IgA nephropathy emerges from a multi-hit hypothesis in which aberrant galactose-deficient IgA1 production triggers the formation of circulating immune complexes. These complexes deposit in the glomerular mesangium, eliciting a cascade of inflammatory and fibrotic responses. Over time, persistent immune activation contributes to mesangial proliferation, matrix expansion, and eventual glomerulosclerosis. Furthermore, genetic predisposition and environmental triggers, such as mucosal infections, converge to amplify the disease trajectory, underscoring the complexity of its underlying biology.
Clinically, the presentation spans a spectrum from isolated microscopic hematuria to rapidly progressive glomerulonephritis. A substantial proportion of patients exhibit indolent proteinuria and preserved renal function for years, while others progress swiftly to end-stage renal disease. This heterogeneity complicates risk stratification and management decisions, demanding a nuanced approach to monitoring and therapeutic intervention. Standard prognostic tools, including renal biopsy scoring and clinical biomarkers, provide valuable data but often fall short in guiding personalized treatment.
Despite notable advances in understanding disease mechanisms, therapeutic strategies remain largely supportive, focusing on blood pressure control, reduction of proteinuria, and off-label use of immunosuppressants. The absence of approved targeted therapies and the reliance on regimens associated with toxicity represent significant unmet needs. In this context, continued research toward novel agents and precision medicine approaches is essential to transform the landscape of IgA nephropathy care.
Revolutionary Advances in Diagnostics Molecular Therapies and Patient-Centric Strategies Reshaping the IgA Nephropathy Treatment Paradigm
In recent years, the landscape of IgA nephropathy has been reshaped by breakthroughs in molecular characterization and diagnostic precision. Advances in high-throughput sequencing have illuminated genetic variants linked to disease susceptibility, while proteomic and metabolomic profiling have uncovered novel biomarkers that correlate with disease activity. These scientific strides have enabled clinicians to detect early pathological changes with greater sensitivity, moving beyond traditional reliance on invasive biopsy procedures and broad clinical markers.Concurrently, the identification and validation of surrogate endpoints have accelerated clinical development. Biomarkers such as urinary CD89 and serum levels of galactose-deficient IgA1 now inform risk stratification and facilitate patient selection in clinical trials. Integration of multi-omic data streams has fostered a more holistic understanding of disease pathogenesis, guiding the design of targeted interventions and enabling adaptive trial models that prioritize efficiency without compromising rigor.
The therapeutic pipeline has also witnessed transformative innovation. Complement inhibitors, B-cell modulators, and agents targeting key cytokine pathways are emerging from late-stage clinical evaluation. Monoclonal antibodies designed to neutralize pathogenic immune complexes and small molecules aimed at modulating mesangial signaling exemplify a shift toward mechanism-based therapies. This movement heralds the potential for interventions that not only slow disease progression but also address underlying immune dysregulation.
Regulatory frameworks have evolved in parallel, with several authorities establishing expedited pathways for rare and progressive renal diseases. Clearer guidance on surrogate endpoints and conditional approval mechanisms has emboldened sponsors to pursue ambitious development plans. Such policies underscore a growing recognition of the urgent need for disease-modifying treatments in IgA nephropathy.
Moreover, patient-centric initiatives are gaining traction. Digital health platforms and remote monitoring solutions are being piloted to capture real-world data on treatment response and quality of life. Engagement with patient advocacy groups has informed clinical trial design, ensuring that outcomes align with patient priorities. Together, these transformative shifts promise to redefine both the conceptual framework and practical management of IgA nephropathy.
Assessing the Comprehensive Economic and Supply Chain Implications of Newly Implemented Tariffs on IgA Nephropathy Therapeutics in the United States 2025
In mid-2025, the implementation of novel tariff measures on imported pharmaceutical ingredients and biologics in the United States introduced a layer of complexity to the supply chain supporting IgA nephropathy treatments. These duties, applied to key active pharmaceutical ingredients and components used in manufacturing monoclonal antibodies, have raised production costs for both established and emerging therapies. As a result, manufacturers face pressure to adjust pricing strategies, negotiate with payers, or absorb margins, all of which have implications for patient access and reimbursement.This shift has engendered strategic responses across the industry. Some organizations have accelerated efforts to diversify their supplier base, establishing relationships with domestic and nearshore contract manufacturers to mitigate duties on imported materials. Others have invested in in-house capacity expansion, seeking greater control over critical supply chain nodes and reducing vulnerability to external tariff fluctuations. These approaches, while resource-intensive, aim to ensure continuity of supply and stabilize cost structures over the long term.
Payers and healthcare providers are closely monitoring these developments. Increased production costs may translate into higher list prices or more stringent utilization management practices. Negotiation dynamics have shifted, with payers leveraging potential tariff-related cost increases to secure value-based contracts or outcomes-based pricing agreements. In parallel, some providers are exploring alternative treatment pathways, including generics or biosimilars, to maintain affordability.
Patient advocacy groups have underscored the risk of treatment delays and access barriers, particularly for individuals reliant on specialized infusion centers or home infusion services. Real-world implications include extended waiting times for critical therapies and variability in coverage across payor networks. Furthermore, digital traceability tools and advanced inventory management systems are being adopted to track material origins and optimize stock levels. By leveraging analytics on import patterns and duty thresholds, manufacturers and distributors aim to forecast tariff exposure and plan procurement cycles accordingly.
In this environment of evolving cost pressures, collaboration among stakeholders has become increasingly important. Consortiums that bring together biopharmaceutical companies, logistics partners, and healthcare delivery organizations are exploring shared infrastructure models to centralize warehousing and distribution. Such cooperative frameworks can yield economies of scale, spreading tariff-related risks and fostering resilience in the supply network.
Overall, the cumulative impact of the 2025 tariff landscape is redefining cost structures and operational paradigms in the IgA nephropathy therapeutic arena. The industry's ability to harness strategic sourcing, supply chain diversification, and digital innovation will determine the extent to which patient access and care quality are preserved in this dynamic economic context.
Drug Class Treatment Modalities Patient Types End Users and Distribution Channels Reveal Critical Dynamics in IgA Nephropathy Management
Analysis of the drug class segmentation reveals a diverse therapeutic arsenal for IgA nephropathy, with renin-angiotensin system inhibitors playing a foundational role. Agents such as ACE inhibitors and angiotensin receptor blockers remain mainstays for controlling hypertension and mitigating proteinuria. In parallel, the category of immunosuppressants has been further dissected to include classical agents like azathioprine, corticosteroids and cyclophosphamide alongside newer options such as mycophenolate mofetil. These variations in immunosuppressive regimens reflect efforts to balance efficacy against tolerability. Additionally, recent entry of monoclonal antibodies designed to neutralize pathogenic immune complexes signals a paradigm shift toward targeted biologic interventions.When considering treatment modalities, a spectrum emerges from supportive care in renal replacement settings to curative intents. Dialysis continues to serve as a vital lifeline for patients advancing to end-stage renal disease, while kidney transplantation offers the potential for longer-term disease resolution. Immunosuppressive therapy remains central in moderating disease activity, complemented by non-immunosuppressive approaches aimed at reducing glomerular hyperfiltration. Plasmapheresis is occasionally employed in refractory or rapidly progressive cases, underscoring the need for tailored therapeutic pathways.
Within the patient landscape, adults represent the predominant cohort, yet elderly patients often present with comorbidities that influence risk-benefit assessments. Pediatric patients, though smaller in number, require specialized dosing and monitoring strategies to address growth and developmental considerations. Each patient type demands a nuanced approach that accounts for age-related pharmacodynamics and long-term outcome goals.
End users drive service delivery in varied settings, ranging from home healthcare environments to tertiary hospitals and dedicated research institutes. Specialty clinics further refine patient management through protocolized care pathways. In turn, distribution channels encompass both offline pharmacies-spanning hospital and retail outlets-and digital dispensing via online pharmacies, with each channel shaping access, inventory management and patient adherence dynamics.
Comparative Assessment of Americas Europe Middle East and Africa and Asia Pacific Revealing Regional Drivers Shaping IgA Nephropathy Care Pathways
In the Americas, the management of IgA nephropathy is characterized by robust clinical infrastructures and well-established reimbursement frameworks. The United States and Canada benefit from comprehensive insurance coverage models that accommodate advanced therapeutics, though variability in formulary access and prior authorization processes can introduce barriers. Latin American markets, while demonstrating growing interest in novel agents, continue to navigate challenges related to resource allocation and healthcare equity, prompting regional initiatives aimed at capacity building and access optimization.Europe, Middle East and Africa present a mosaic of regulatory and economic environments. In Western Europe, centralized and national authorities maintain clear pathways for drug approval, facilitating timely introduction of innovative therapies. Central and Eastern European markets often contend with budget constraints, leading to tiered access models and selective adoption of high-cost biologics. In the Middle East, strategic investments in healthcare infrastructure have enabled the deployment of specialized nephrology services, whereas African nations face limitations in diagnostic capabilities and specialist availability, necessitating international partnerships to bolster local capacity.
Asia-Pacific stands at the forefront of emerging trends in IgA nephropathy research and treatment. East Asian countries, particularly China, Japan and South Korea, exhibit high disease prevalence and have prioritized domestic development of targeted therapies. These markets benefit from government-sponsored research funding and streamlined regulatory processes for rare renal diseases. Southeast Asian and Oceanic regions are increasingly engaging with global clinical trials and expanding access to monoclonal antibody platforms. Collaboration between multinational and local companies is catalyzing market entry strategies that align with regional pricing and health technology assessment requirements.
Collectively, regional dynamics underscore the importance of tailored approaches to regulatory engagement, reimbursement negotiation and stakeholder education, each of which shapes the trajectory of patient care across diverse geographies.
Strategic Positioning and Competitive Strengths of Leading Biopharmaceutical Innovators Driving Progress in IgA Nephropathy Therapeutic Development
Leading players in the IgA nephropathy space are leveraging diverse strategies to cement their positions and accelerate therapeutic innovation. Large biopharmaceutical corporations with established portfolios in nephrology are fortifying pipelines through targeted acquisitions and strategic alliances. These entities harness their global manufacturing and distribution networks to scale promising candidates rapidly, while also navigating complex patent landscapes to optimize intellectual property protections.At the same time, specialist biotechnology firms are making significant inroads by focusing exclusively on mechanism-based therapies. Organizations advancing complement inhibitors and B-cell modulators have attracted substantial venture backing, enabling them to drive early-stage research with agility. Their nimbleness in trial design and patient engagement offers a competitive edge in refining dosing paradigms and elucidating biomarkers of response.
Collaborative models are also gaining prominence. Partnerships between pharmaceutical companies and academic research institutes have yielded translational platforms that expedite the validation of novel targets. Joint ventures centered on real-world evidence generation accelerate post-approval studies and deepen understanding of long-term outcomes. These cooperative frameworks contribute to a more integrated ecosystem, where knowledge transfer and resource sharing enhance overall therapeutic development efficiency.
Within this landscape, boutique clinical research organizations are emerging as critical enablers, providing specialized trial management and patient recruitment capabilities. Their regional expertise ensures that diverse patient populations are represented, fostering data integrity and regulatory compliance. Collectively, the interplay of large-scale players, entrepreneurial biotechs and collaborative networks defines the competitive contours of the IgA nephropathy market, guiding the pace at which innovative treatments reach clinical practice.
Strategic Initiatives for Industry Stakeholders to Enhance Innovation Collaboration and Patient Access in the Evolving IgA Nephropathy Therapeutic Landscape
To navigate the evolving IgA nephropathy ecosystem effectively, industry leaders should prioritize initiatives that bridge scientific innovation and real-world applicability. Strengthening biomarker-driven clinical trial designs will enhance patient selection and improve the likelihood of demonstrating meaningful clinical benefits. By integrating genomic, proteomic and imaging data early in developmental stages, sponsors can reduce variability and accelerate decision-making processes.Supply chain resilience must also be bolstered in light of recent tariff and regulatory shifts. Establishing diversified sourcing strategies and fostering partnerships with domestic manufacturing facilities can mitigate exposure to external economic fluctuations. Concurrently, investing in digital inventory management platforms will provide real-time visibility into material flows and enable proactive risk management.
Engagement with payer organizations should commence at the earliest planning phases. Collaborative value demonstration programs that emphasize health economics and patient-reported outcomes lay the groundwork for favorable reimbursement frameworks. Outcomes-based contracting models, enriched by robust real-world evidence, can align stakeholder incentives and ensure sustainable access.
Finally, fostering patient engagement throughout the product lifecycle is essential. Collaborating with advocacy groups to design trials and develop educational initiatives will enhance recruitment rates and support adherence. Digital health solutions that offer remote monitoring and telehealth consultations can improve patient experience and provide continuous data streams for iterative therapeutic optimization. By executing these actionable strategies, industry stakeholders will be well-positioned to deliver transformative solutions for individuals living with IgA nephropathy.
Rigorous Multi-Modal Qualitative and Quantitative Framework Leveraging Expert Insights Data Triangulation and Validation for Comprehensive Market Understanding
Research for this executive summary was conducted through a rigorous multi-modal approach combining primary and secondary data sources. Initial efforts focused on a comprehensive literature review encompassing peer-reviewed journals, clinical trial registries and regulatory guidelines. This foundational analysis established the scientific and clinical context necessary to frame subsequent inquiries.Primary research involved in-depth interviews with key opinion leaders, including nephrologists, pharmacologists and health economics experts, to capture nuanced perspectives on current challenges and emerging opportunities. These conversations provided valuable insights into clinical practice variations, trial design considerations and payer negotiation strategies. Interview data were systematically collated and coded to identify recurring themes and divergent viewpoints.
Secondary data collection included examination of industry reports, patent filings and corporate disclosures, offering visibility into competitive dynamics and pipeline progress. Data triangulation techniques were employed to cross-validate findings, ensuring consistency between qualitative insights and documented evidence. Where discrepancies arose, follow-up consultations with subject matter experts facilitated clarification and reinforced analytical rigor.
Validation protocols entailed peer review by an external advisory panel with representation from clinical, regulatory and market access domains. Feedback from this panel informed iterative refinements, enhancing the credibility and relevance of conclusions. Ethical considerations and confidentiality agreements were strictly observed throughout, guaranteeing that proprietary information was managed responsibly. This methodical framework underpins the strategic recommendations and ensures that the analysis reflects a balanced and authoritative view of the IgA nephropathy landscape.
Synthesis of Emerging Themes Unmet Challenges and Pathways Forward in Advancing Patient-Centric Solutions for IgA Nephropathy Across Stakeholders
As the landscape of IgA nephropathy continues to evolve, it is clear that an integrated approach embracing scientific innovation, regulatory agility and stakeholder collaboration is essential. The progression from broad immunosuppressive regimens to targeted biologic therapies underscores the transformative potential of mechanism-based interventions. Concurrent advances in biomarker discovery and digital health solutions further enhance the ability to personalize care and improve patient outcomes.However, challenges remain. Complex supply chains, emerging economic pressures and heterogeneity in regional healthcare systems necessitate resilient strategies that align manufacturers, providers and payers. Addressing these challenges requires a concerted effort to harmonize regulatory pathways, validate meaningful surrogate endpoints and demonstrate real-world value. Equally important is the need to foster patient engagement, ensuring that clinical development plans reflect the priorities and lived experiences of those affected by this condition.
The insights presented in this executive summary highlight critical inflection points for industry stakeholders. By capitalizing on collaborative frameworks and leveraging data analytics, organizations can navigate the shifting dynamics of tariffs, segmentation and regional landscapes. Ultimately, the path forward hinges on a shared commitment to innovation and value creation, propelling the field toward therapeutic breakthroughs that will redefine standards of care for individuals living with IgA nephropathy.
Looking ahead, the integration of artificial intelligence and machine learning into clinical and operational processes promises to further optimize trial design, patient recruitment and supply chain management. Continual investment in these areas will cement the foundation for sustained progress. In sum, the confluence of scientific advances, technology adoption and strategic partnerships positions the industry to meet the unmet needs of IgA nephropathy patients worldwide.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Drug Class
- ACE Inhibitors
- Angiotensin Receptor Blockers
- Immunosuppressants
- Azathioprine
- Corticosteroids
- Mycophenolate mofetil
- Treatment Type
- Immunosuppressive Therapy
- Plasmapheresis
- Renal Replacement Therapies
- Dialysis
- Kidney Transplantation
- Supportive Care
- Route Of Administration
- Intravenous
- Oral
- Subcutaneous
- Formulation
- Capsule
- Injection
- Oral Solution
- Tablet
- Patient Type
- Adults
- Elderly Patients
- Pediatric Patients
- End User
- Academic & Research Institutes
- Home Care Settings
- Hospitals
- Specialty Clinics/Nephrology Clinics
- Distribution Channel
- Offline Pharmacies
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Offline Pharmacies
- Indication
- Primary IgA Nephropathy
- Secondary IgA Nephropathy
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Alnylam Pharmaceuticals, Inc.
- Apellis Pharmaceuticals Inc.
- Biogen Inc.
- Boehringer Ingelheim International GmbH.
- Calliditas Therapeutics AB by Asahi Kasei Corporation
- ChemoCentryx, Inc. by Amgen Inc.
- Eledon Pharmaceuticals Inc.
- Ionis Pharmaceuticals Inc
- Keymed Biosciences Inc.
- Novartis AG
- NovelMed Inc.
- Novo Nordisk A/S.
- Omeros Corporation
- Otsuka Pharmaceutical Co. Ltd
- Q32 Bio Inc.
- Takeda Pharmaceutical Company
- Travere Therapeutics Inc
- Vera Therapeutics Inc
- Vertex Pharmaceuticals Incorporated
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this IgA Nephropathy market report include:- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Alnylam Pharmaceuticals, Inc.
- Apellis Pharmaceuticals Inc.
- Biogen Inc.
- Boehringer Ingelheim International GmbH.
- Calliditas Therapeutics AB by Asahi Kasei Corporation
- ChemoCentryx, Inc. by Amgen Inc.
- Eledon Pharmaceuticals Inc.
- Ionis Pharmaceuticals Inc
- Keymed Biosciences Inc.
- Novartis AG
- NovelMed Inc.
- Novo Nordisk A/S.
- Omeros Corporation
- Otsuka Pharmaceutical Co. Ltd
- Q32 Bio Inc.
- Takeda Pharmaceutical Company
- Travere Therapeutics Inc
- Vera Therapeutics Inc
- Vertex Pharmaceuticals Incorporated
Table Information
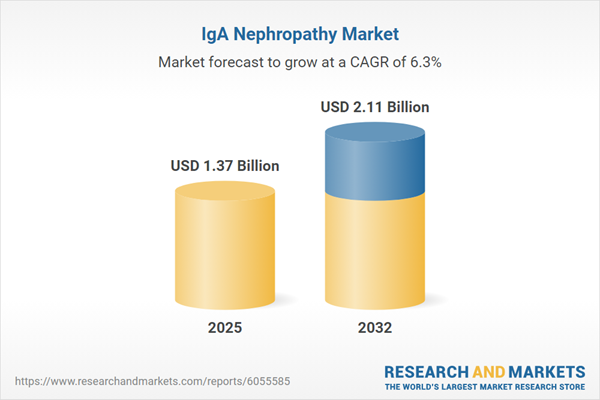
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.37 Billion |
| Forecasted Market Value ( USD | $ 2.11 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |