Speak directly to the analyst to clarify any post sales queries you may have.
The GMP Grade Cell Culture Media Market is experiencing sustained expansion, fueled by innovation in bioprocessing and growing regulatory focus. Senior decision-makers in biopharmaceuticals and manufacturing are increasingly reliant on standardized, high-quality media to advance next-generation therapies, ensuring both regulatory compliance and operational efficiency.
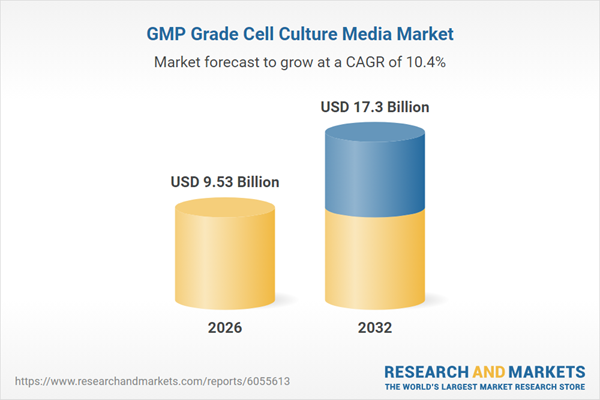
Market Snapshot: GMP Grade Cell Culture Media Market Overview
The GMP grade cell culture media market is valued at USD 8.67 billion in 2025, projected to reach USD 9.53 billion in 2026, and is expected to continue growing at a CAGR of 10.36% to USD 17.30 billion by 2032. This growth reflects increased adoption in advanced biologics, monoclonal antibodies, and cell and gene therapies, highlighting the market’s critical role in therapeutic manufacturing pipelines.
Scope & Segmentation
- Product Types: Chemically defined media, classical media, serum-free options such as specialized CHO and VERO cell media, and niche specialty formulations, each designed to meet distinct bioprocessing specifications.
- Component Scope: Key raw materials include amino acids for protein synthesis, growth factors for cell proliferation, hormones to modulate function, trace elements supporting enzymatic activities, and a spectrum of vitamins essential for metabolic pathways.
- Form: Both liquid and powder formats are available, with liquid media facilitating immediate use and powder media offering logistical advantages for scalable operations.
- Cell Type: Tailored media formulations address specific needs for mammalian, bacterial, insect, avian, and yeast cell cultures, supporting viability and productivity.
- Application Areas: Encompasses production of monoclonal antibodies, recombinant proteins, vaccines, and advanced areas such as cell and gene therapies, as well as robust applications in drug discovery and in vitro modeling.
- Regional Coverage: Americas, Europe, Middle East and Africa, and Asia Pacific, with each region reflecting unique adoption drivers rooted in technology leadership, regulatory landscape, production capacity, and local manufacturing investments.
- Technology Drivers: Integration of digital analytics, process automation, and modular manufacturing platforms underpin process monitoring, quality assurance, and adaptive control.
- Sustainability Shifts: Movement toward chemically defined, animal component-free media supports both ethical considerations and consistency in product outcomes.
Key Takeaways for Senior Decision-Makers
- GMP compliant cell culture media is a strategic enabler, supporting reproducibility, efficient scale-up, and consistent product quality across diverse therapeutic platforms.
- Technological convergence, including continuous bioprocessing and automation, is reshaping formulation and production, driving higher yields and process reliability.
- Suppliers are reengineering portfolios with application-specific and customizable media solutions, particularly for sensitive cell types required in emerging gene and cell therapies.
- Sustainability goals are leading to greater adoption of animal component-free media and ethical sourcing, aligning product strategies with regulatory and societal expectations.
- Adoption of advanced analytics facilitates real-time process adjustments, minimizing variance and reducing risk throughout manufacturing operations.
- Regional market maturity, logistics, and manufacturing infrastructure significantly influence supplier strategies and partnership models.
Tariff Impact: Navigating Trade Fluctuations
The introduction of new United States tariff policies in 2025 has altered global procurement dynamics for GMP grade cell culture media, influencing raw material costs and shaping future supply chain strategies. Manufacturers are responding by expanding domestic partnerships and exploring nearshoring to address cost and risk management, although these shifts require substantial investment and adaptation. As documentation aligns with updated trade classifications, organizations are urged to strengthen risk mitigation and flexible planning across their supply chains.
Methodology & Data Sources
This report draws from a multi-phase research methodology, beginning with comprehensive reviews of peer-reviewed literature and regulatory filings. In-depth interviews with industry leaders—including bioprocess engineers, media formulators, and regulatory experts—provided grounded insights into formulation, sourcing, and innovation developments. Continuous feedback loops and scenario testing ensured robust validation of all presented trends and projections.
Why This Report Matters
- Enables strategic alignment of R&D, manufacturing, and investment priorities with the most dynamic growth areas in the GMP grade cell culture media market.
- Offers actionable analysis to mitigate risks related to supply chain shifts, regulatory evolution, and tariff-induced cost pressures.
- Equips stakeholders with high-confidence insights needed for targeted expansion, sustainable innovation, and portfolio optimization in an evolving landscape.
Conclusion
Strategic adoption of GMP grade cell culture media that leverages advanced technology, robust supply chains, and sustainable practices is critical to maintaining competitiveness. This analysis provides the framework for industry leaders to align innovation, compliance, and market expansion for future success.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
- ACROBIOSYSTEMS INC
- Avantor, Inc.
- Becton, Dickinson and Company
- Bio-Techne Corporation
- BioLife Solutions Inc.
- Capricorn Scientific GmbH
- Cook Group Incorporated
- Corning Incorporated
- Danaher Corporation
- Elabscience Bionovation Inc.
- FUJIFILM Holdings Corporation
- Gemini BioProducts LLC
- HiMedia Laboratories Pvt. Ltd
- InVitria
- Lonza Group Ltd.
- Merck KGaA
- Miltenyi Biotec B.V. & Co. KG
- MP Biomedicals, LLC
- PAN-Biotech GmbH
- Plant Cell Technology Inc
- PromoCell GmbH
- Sartorius AG
- Shanghai BioEngine Sci-Tech Co., Ltd.
- STEMCELL Technologies Canada Inc.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 9.53 Billion |
| Forecasted Market Value ( USD | $ 17.3 Billion |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |