Speak directly to the analyst to clarify any post sales queries you may have.
Awakening to the Critical Role of Recombinant Uricase Therapeutics in Modern Healthcare and Its Promise for Transformational Disease Management
The emergence of recombinant uricase represents a pivotal advance in therapeutic innovation, carrying the promise of transforming the treatment landscape for hyperuricemia and related disorders. This class of enzyme therapy, optimized through precision bioengineering, has evolved into a cornerstone modality for addressing uric acid metabolism challenges where conventional therapies often fall short. In today's complex pharmaceutical environment, the need for targeted solutions that can efficiently catalyze uric acid degradation has never been more acute, particularly given rising global incidences of gout, tumor lysis syndrome, and rare genetic conditions marked by excessive uric acid accumulation.Building a comprehensive understanding of recombinant uricase involves recognizing its multifaceted potential, from non-pegylated formulations that offer rapid enzyme activity to pegylated variants delivering extended half-life benefits and improved patient compliance. Moreover, biosimilar developments are reshaping competitive dynamics by enhancing accessibility while driving cost efficiencies. As these therapies advance through clinical pipelines and gain regulatory approvals, stakeholders across healthcare delivery, pharmaceutical manufacturing, and research institutes must adapt to the converging influences of molecular innovation, patient demand, and evolving reimbursement models.
Consequently, this introduction sets the stage for a deeper exploration of the transformative shifts, tariff impacts, segmentation dynamics, regional nuances, and strategic imperatives that will define the recombinant uricase market over the coming years. By analyzing these dimensions with rigor and foresight, decision-makers will be better equipped to harness innovation pathways and optimize therapeutic outcomes for patients worldwide.
Unprecedented Technological and Regulatory Shifts Reshaping the Recombinant Uricase Market Landscape and Accelerating Next Generation Treatments
In recent years, the recombinant uricase market has witnessed unprecedented technological breakthroughs and regulatory reforms that are collectively reshaping how these enzyme therapies are developed, manufactured, and delivered. Advances in pegylation chemistry, for instance, have extended circulating half-life and reduced immunogenicity, enabling less frequent dosing schedules and improved patient adherence. At the same time, novel expression systems leveraging plant and microbial hosts are driving enhanced yield efficiencies and scalable production compared to traditional mammalian cell lines.Regulatory agencies across key regions have responded to these innovations by implementing expedited review pathways and offering adaptive licensing frameworks for orphan and breakthrough therapies. These measures are catalyzing a faster transition from bench to bedside, allowing patients with refractory gout, tumor lysis syndrome, and rare genetic disorders to access critical treatments sooner. Alongside these approvals, real-world evidence initiatives and patient-centric health technology assessments are enriching data on long-term safety and cost effectiveness, thereby strengthening the value proposition for recombinant uricase therapies among payers and healthcare providers.
In parallel, digital health platforms and advanced analytics are facilitating precision dosing and remote monitoring, empowering clinicians to tailor treatment regimens based on pharmacokinetic modeling and patient feedback. Transitionally, this confluence of scientific innovation, regulatory flexibility, and digital integration is not only accelerating clinical adoption but also driving competitive differentiation. This section uncovers how these transformative shifts are converging to redefine the future of recombinant uricase and establish new benchmarks for therapeutic success.
Understanding the Far Reaching Consequences of the 2025 United States Tariffs on Recombinant Uricase Supply Chains and Industry Economics
The introduction of 2025 tariffs by the United States has exerted wide ranging effects on the global supply chains for recombinant uricase, creating ripple effects that extend from raw material sourcing to final product pricing. Manufacturers dependent on imported biocatalysts and specialized reagents have encountered increased costs that, in turn, impact production economics. As some facilities recalibrate procurement strategies to mitigate duty burdens, alternative suppliers within domestic and allied markets have emerged, albeit often at a premium. Consequently, companies are reassessing their global footprint, evaluating whether to relocate certain production stages or invest in regional partnerships to safeguard supply continuity.Moreover, the tariffs have prompted strategic dialogues between pharmaceutical firms and material vendors aimed at renegotiating long-term contracts and adjusting price structures. These measures are crucial for preserving margin stability while complying with evolving trade regulations. As duties fluctuate, organizations have also intensified efforts to innovate process efficiencies, such as adopting single-use bioreactor technologies that reduce capital expenditure and minimize cross-contamination risks.
Concurrently, shifting cost dynamics have influenced negotiations with payers and providers, underscoring the importance of demonstrating clear clinical value to justify premium pricing. In response, drug developers are deepening pharmacoeconomic studies and generating real-world data to reinforce the health economic benefits of recombinant uricase therapies. Ultimately, the cumulative impact of these tariff measures is driving greater resilience across the supply chain and fostering adaptive strategies that will guide market participants through an increasingly complex trade environment.
Revealing Critical Segmentation Perspectives by Product Type Administration Route Application and End User for Tailored Strategic Initiatives
Deep insights emerge when evaluating the recombinant uricase market through critical segmentation lenses that encompass product type, route of administration, application, and end user. In the realm of product type, differentiation between biosimilars of recombinant uricase, non-pegylated uricase, and pegylated uricase reveals distinct development pathways and clinical profiles. Biosimilar entrants leverage established reference molecules to drive affordability, whereas non-pegylated formulations aim for immediate enzyme activity, and pegylated versions prioritize extended half-life and dosing convenience.Transitioning to route of administration, the choice between intramuscular, intravenous, and subcutaneous delivery shapes patient experience and clinical deployment. Intravenous infusion often suits acute care settings, while subcutaneous options are gaining favor for outpatient and self-administration models. Intramuscular injections, though less common, can serve as alternative approaches when rapid enzyme delivery is required in resource-limited environments.
Applications span cancer treatment, gout management, kidney disease intervention, neurological disorder research, and rare genetic disorder therapies. Within the gout treatment segment, further granularity is evident across chronic gout, hyperuricemia, refractory gout, and tumor lysis syndrome, each demanding tailored dosing regimens and safety considerations. End-user segmentation among healthcare providers, pharmaceutical manufacturers, and research and development institutes highlights diverse stakeholder needs. Clinics, dialysis centers, and hospitals, as subsets of healthcare providers, demonstrate varying infrastructure capabilities and patient throughput, influencing product selection and support services.
These intersecting segments guide strategic investment priorities, clinical trial designs, and commercialization plans. By aligning product portfolios with specific usage scenarios and stakeholder requirements, companies can optimize resource allocation and enhance market penetration.
Exploring Distinct Regional Dynamics across the Americas Europe Middle East Africa and Asia Pacific Driving Diverse Recombinant Uricase Adoption Patterns
Regional dynamics are pivotal in shaping recombinant uricase adoption patterns and strategic opportunities across three major zones: the Americas, Europe Middle East & Africa, and Asia Pacific. In the Americas, robust healthcare infrastructure and progressive reimbursement frameworks foster rapid uptake of advanced biologics. The United States, as a leader, benefits from established regulatory pathways for breakthrough therapies and significant private sector investment in precision medicine initiatives.Across Europe, Middle East & Africa, heterogeneous regulatory landscapes and variable healthcare funding models create a patchwork of market conditions. Western European countries often offer favorable incentives for orphan drug development and strong patient support programs, whereas emerging markets in the Middle East and Africa require targeted infrastructure investments and educational campaigns to enhance clinician proficiency and patient awareness.
The Asia Pacific region, characterized by rapid economic growth and expanding middle-class populations, presents both opportunities and complexities. Countries such as China, Japan, and Australia are advancing regulatory harmonization efforts to streamline biologic approvals, yet local manufacturing incentives and evolving intellectual property frameworks necessitate nuanced market entry strategies. Furthermore, disparities in healthcare access and distribution networks across Southeast Asia and the Pacific Islands require tailored engagement models that address logistical constraints and diverse payer mechanisms.
By interpreting these regional nuances, stakeholders can refine distribution channels, forge strategic alliances with local entities, and calibrate market access strategies. This geographic perspective ensures that recombinant uricase therapies reach patients efficiently while maximizing commercial potential in each territory.
Analyzing Competitive Strengths Strategic Portfolios and Innovation Pipelines of Leading Players in the Recombinant Uricase Sector
Leading players in the recombinant uricase market demonstrate a spectrum of strategic approaches that underscore the competitive landscape's intensity. Innovative biopharmaceutical companies are channeling research and development resources into next-generation pegylation technologies, advanced delivery mechanisms, and novel molecule modifications designed to enhance safety profiles and therapeutic efficacy. Some organizations are forging collaborations with specialized contract manufacturing organizations to leverage state-of-the-art single-use bioreactors, thereby accelerating scale-up and ensuring flexible capacity for clinical and commercial batches.Strategic partnerships between prominent drug manufacturers and academic or research institutions are also fueling pipeline diversification. These alliances enable access to cutting-edge molecular designs and foster co-development models that distribute R&D risk. Meanwhile, emerging biosimilar entrants are leveraging cost advantages and streamlined regulatory processes to establish a foothold in markets with heightened price sensitivity.
Additionally, companies are prioritizing patient support programs, digital adherence tools, and comprehensive training for healthcare providers to differentiate their offerings. By bundling these services with product launches, firms can enhance patient outcomes, reduce administration errors, and build stronger relationships with key stakeholders. Across the competitive spectrum, the emphasis on strategic collaborations, manufacturing excellence, and patient-centric support underscores the multifaceted efforts driving the recombinant uricase arena forward.
Strategic Imperatives and Proactive Measures for Industry Leaders to Capitalize on Emerging Opportunities in Recombinant Uricase Development
Industry leaders must adopt a multifaceted strategy that balances innovation, operational agility, and stakeholder engagement to maintain competitive advantages in the recombinant uricase landscape. First, investment in targeted research platforms that explore novel pegylation methods and alternative expression systems will be critical for driving next-generation therapies. By reallocating resources toward modular manufacturing capabilities, organizations can respond more swiftly to regulatory changes and minimize capital lock-in.Simultaneously, forging strategic alliances with regional partners and contract service providers can mitigate supply chain risks heightened by trade dynamics and tariff fluctuations. Proactive inventory management and dual-sourcing arrangements will enhance resilience while supporting uninterrupted product availability. In parallel, deepening collaborations with patient advocacy groups and professional societies will enrich real-world data generation and bolster pharmacoeconomic evidence, strengthening payer negotiations and expanding market access.
Moreover, embedding digital health solutions such as remote monitoring platforms and AI-driven adherence tools into commercialization plans will empower clinicians and patients, fostering improved therapeutic outcomes. Equally important is the cultivation of robust patient support infrastructures, including education initiatives and reimbursement navigation services, which can differentiate offerings and drive long-term loyalty. Collectively, these actionable measures will enable industry stakeholders to capitalize on evolving market opportunities and sustain momentum in recombinant uricase innovation.
Employing Rigorous Multidimensional Research Approaches and Analytical Frameworks to Illuminate the Recombinant Uricase Market with High Confidence
To ensure comprehensive market intelligence, this research employed a robust methodology that integrates both primary and secondary data sources. Primary research involved in-depth interviews with key opinion leaders, industry executives, and clinical specialists to obtain qualitative insights on emerging trends, patient needs, and regulatory expectations. These conversations were complemented by detailed surveys among healthcare providers and pharmaceutical manufacturers to validate hypothesis and enrich quantitative analysis.Secondary research comprised systematic reviews of scientific literature, regulatory filings, patent databases, and industry white papers. This phase included a thorough examination of clinical trial registries, government reports, and peer-reviewed publications to contextualize therapeutic advancements and assess competitive activity. Analytical frameworks such as SWOT assessments, Porter's Five Forces, and value chain analysis were applied to evaluate market drivers, challenges, and strategic opportunities.
Data triangulation techniques were employed to reconcile information across sources and enhance the reliability of insights. Furthermore, regional validation workshops were conducted with local stakeholders to calibrate findings against market realities and ensure that cultural, economic, and regulatory nuances were accurately captured. This multidimensional approach underpins the report's conclusions and provides a rigorous foundation for strategic decision-making in the recombinant uricase sector.
Synthesizing Insights to Chart a Forward Looking Path for Recombinant Uricase Innovation Collaboration and Market Growth
The synthesis of these insights reveals a dynamic landscape for recombinant uricase characterized by advancing molecular innovations, evolving regulatory environments, and shifting commercial priorities. As technological breakthroughs in pegylation and expression systems continue to emerge, stakeholders must remain vigilant to leverage these developments while navigating tariff influences and competitive pressures. Strategic alignment across product segmentation, regional dynamics, and patient-centric support models will be essential for unlocking sustainable growth.Looking ahead, the confluence of digital health integration, real-world evidence generation, and collaborative partnerships will define success in this space. Organizations that proactively adapt manufacturing strategies, strengthen supply chain resilience, and deepen stakeholder engagement will be best positioned to deliver high-impact therapies and drive meaningful outcomes for patients living with gout, tumor lysis syndrome, and rare genetic disorders.
Ultimately, the recombinant uricase market offers substantial prospects for innovation and value creation. By harnessing the insights outlined in this executive summary and committing to strategic agility, industry participants can chart a forward-looking path that accelerates therapeutic progress and enhances patient lives.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Biosimilars of Recombinant Uricase
- Non-Pegylated Uricase
- Pegylated Uricase
- Route Of Administration
- Intramuscular
- Intravenous
- Subcutaneous
- Application
- Cancer Treatment
- Gout Treatment
- Chronic Gout
- Hyperuricemia
- Refractory Gout
- Tumor Lysis Syndrome
- Kidney Diseases
- Neurological Disorders
- Rare Genetic Disorders
- End-User
- Healthcare Providers
- Clinics
- Dialysis Centers
- Hospitals
- Pharmaceutical Manufacturers
- Research & Development Institutes
- Healthcare Providers
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3SBio Inc.
- BioCryst Pharmaceuticals, Inc.
- Biosynth Ltd
- Creative Enzymes
- CUSABIO TECHNOLOGY LLC
- GenScript Biotech Corporation
- Hzymes Biotech
- Innovent Biologics, Inc.
- Kikkoman Corporation
- Merck KGaA
- OYC Americas, Inc.
- Protalix BioTherapeutics
- Sisco Research Laboratories Pvt. Ltd.
- Swedish Orphan Biovitrum AB
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Recombinant Uricase market report include:- 3SBio Inc.
- BioCryst Pharmaceuticals, Inc.
- Biosynth Ltd
- Creative Enzymes
- CUSABIO TECHNOLOGY LLC
- GenScript Biotech Corporation
- Hzymes Biotech
- Innovent Biologics, Inc.
- Kikkoman Corporation
- Merck KGaA
- OYC Americas, Inc.
- Protalix BioTherapeutics
- Sisco Research Laboratories Pvt. Ltd.
- Swedish Orphan Biovitrum AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
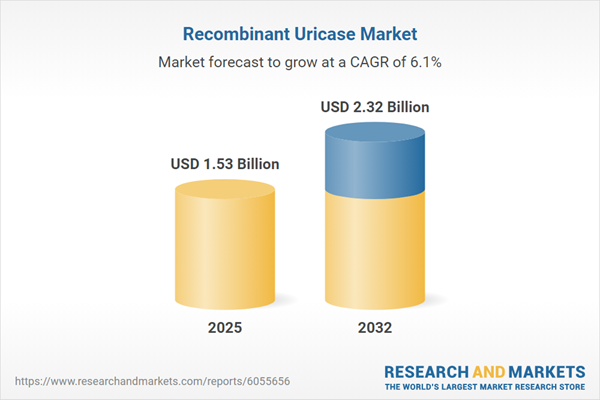
| Estimated Market Value ( USD | $ 1.53 Billion |
| Forecasted Market Value ( USD | $ 2.32 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |