Speak directly to the analyst to clarify any post sales queries you may have.
The Medical Hemodiafiltration Machine Market is evolving rapidly as providers seek to enhance technology adoption and patient care quality across renal therapy programs. This environment requires adaptive strategies from industry leaders.
Market Snapshot: Medical Hemodiafiltration Machine Market Overview
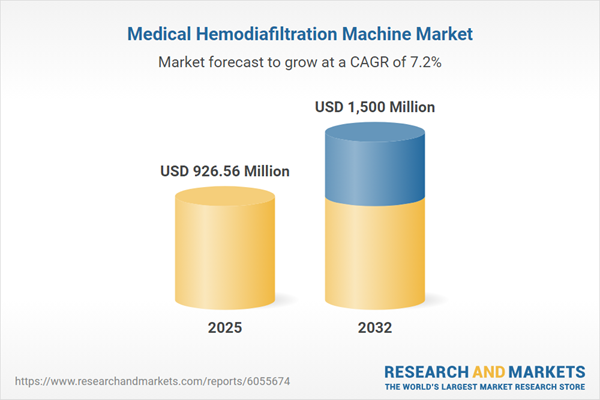
Between 2024 and 2025, the Medical Hemodiafiltration Machine Market is set to expand from USD 866.84 million to USD 926.56 million, projecting continued growth to USD 1.50 billion by 2032 at a CAGR of 7.18%. Market momentum arises from higher global incidences of chronic kidney diseases, deepening technology integration across care environments, and regulatory requirements that drive advancements in device safety and quality. Changing models of renal care are actively shaping adoption patterns throughout developed and emerging healthcare economies, reinforcing the need for innovation and flexibility.
Scope & Segmentation
This report guides senior decision-makers through competitive benchmarking, strategic differentiation, and optimization of initiatives within medical technology. It addresses market diversity with in-depth analysis across major segments and regions as follows:
- Portability: Includes assessment of portable and stationary devices, with a focus on enabling renal therapy beyond traditional medical centers, supporting care in non-hospital and home settings.
- Components: Reviews core modules such as blood pumps, dialyzer units, and hemodiafilters, evaluating their role in ensuring reliability, operational efficiency, and improved patient health outcomes.
- Modes: Analyzes therapy delivery through mid-dilution, postdilution, and predilution HDF modalities, highlighting their value in individualized care planning and clinical outcome optimization.
- End User: Explores usage in hospitals, dialysis clinics, home care environments, and research institutes, emphasizing the prominent shift toward patient-centric service models and their operational implications.
- Regions: Covers the Americas—including the United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, and Peru—where varying reimbursement systems influence adoption strategies. Examines Europe, Middle East, and Africa—such as the United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, and Kenya—as markets experience modernization and regulatory harmonization. Details Asia-Pacific markets—China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, and Taiwan—where population growth and healthcare infrastructure investment drive uptake.
- Companies: Provides strategic profiles of market participants, including ARI Medical Technology Co., Ltd., Asahi Kasei Corporation, AWAK Technologies Pte. Ltd., B. Braun Melsungen AG, Baxter International Inc., DaVita Inc., Diality, Inc., Fresenius Medical Care AG & Co. KGaA, Guangzhou MeCan Medical Limited, Infomed SA, JMS Co. Ltd., Kalstein, KNS MEDICAL SAS, Medtronic PLC, Mozarc Medical Holding LLC, Nephro Care India Limited, Nikkiso Co., Ltd., Nipro Corporation, Outset Medical, Inc., Quanta Dialysis Technologies Ltd., Rockwell Medical, Inc., Shenzhen Landwind Industry Co., Ltd., Soxa Formulations & Research (Pvt.) Ltd., SWS Hemodialysis Care Co., Ltd., Toray Industries, Inc., and Wesley Biotech Co., Ltd.
Key Takeaways for Decision-Makers
- Advancements in device analytics and membrane technology are setting new technical and clinical benchmarks in renal therapy, ensuring safer and more effective treatment delivery.
- Enhanced equipment portability and modular designs support the move to decentralized care models, enabling treatment flexibility and optimizing resource use within healthcare networks.
- Regulatory agencies are focusing on purity and biocompatibility, prompting manufacturers to prioritize continuous improvements in both device safety and workflow integration.
- Strategic partnerships and implementation of advanced digital systems are increasing data accuracy, boosting monitoring capabilities, and improving coordination between clinical teams.
- Custom solutions for hospital and home-based therapies are now standard, enabling providers to address a wide spectrum of operational requirements and patient preferences across diverse care settings.
- Regional discrepancies in infrastructure and reimbursement drive the need for tailored market entry and expansion strategies, with adaptability as a critical success factor.
Tariff Impact on the Medical Hemodiafiltration Machine Market
Recent changes in United States tariff policy have led manufacturers to re-examine sourcing and supply chain configurations for medical hemodiafiltration machines. These adjustments raise component costs and encourage companies to localize supply flows, impacting inventory practices and distribution strategies. Maintaining compliance and optimizing operational costs amid evolving regulations has become a primary concern for leaders seeking to ensure supply chain resilience and timely technology adoption.
Methodology & Data Sources
This analysis merges peer-reviewed secondary data with targeted primary interviews of clinical and supply chain executives. The triangulation approach strengthens accuracy, providing a comprehensive, up-to-date perspective on current practices and recent advancements in the medical device field.
Why This Report Matters
- Empowers leadership teams with actionable segmentation, strengthening decision-making for procurement, investment, and patient care program development.
- Delivers balanced analysis supporting optimized technology selection and supply network design aligned with regulatory and operational shifts.
- Equips senior stakeholders to respond to innovation trends and adjust strategies for effective regional and global market penetration.
Conclusion
This report equips industry leaders with strategic guidance to address regulatory shifts, technology evolution, and varying regional dynamics, providing a reliable foundation for sustained growth and organizational resilience in renal therapy.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Medical Hemodiafiltration Machine market report include:- ARI Medical Technology Co., Ltd.
- Asahi Kasei Corporation
- AWAK Technologies Pte. Ltd.
- B. Braun Melsungen AG
- Baxter International Inc.
- DaVita Inc.
- Diality, Inc.
- Fresenius Medical Care AG & Co. KGaA
- Guangzhou MeCan Medical Limited
- Infomed SA
- JMS Co. Ltd.
- Kalstein
- KNS MEDICAL SAS
- Medtronic PLC
- Mozarc Medical Holding LLC
- Nephro Care India Limited
- Nikkiso Co., Ltd.
- Nipro Corporation
- Outset Medical, Inc.
- Quanta Dialysis Technologies Ltd.
- Rockwell Medical, Inc.
- Shenzhen Landwind Industry Co., Ltd.
- Soxa Formulations & Research(Pvt.) Ltd.
- SWS Hemodialysis Care Co., Ltd.
- Toray Industries, Inc.
- Wesley Biotech Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 926.56 Million |
| Forecasted Market Value ( USD | $ 1500 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |