Speak directly to the analyst to clarify any post sales queries you may have.
The Nanorobots in Healthcare Market is rapidly evolving, providing significant potential for senior leaders aiming to drive improvements in diagnostic accuracy, targeted therapies, and patient-centric monitoring in the field of precision medicine. Early adoption and informed investment in nanorobotic solutions can set organizations apart in operational capability and clinical performance.
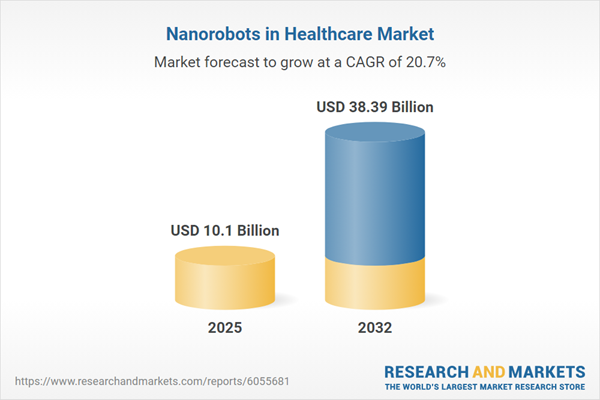
Market Snapshot: Nanorobots in Healthcare Market
Demonstrating substantial momentum, the Nanorobots in Healthcare Market expanded from USD 8.49 billion in 2024 to an estimated USD 10.10 billion in 2025, projecting robust long-term growth to USD 38.39 billion by 2032 and a compound annual growth rate (CAGR) of 20.74%. Drivers of this growth include advances in materials science, breakthroughs in microelectronics, and robust artificial intelligence integration within clinical applications. These technological advancements are enabling minimally invasive procedures and higher-precision medical interventions, supporting healthcare organizations in aligning with emerging clinical protocols and addressing more intricate patient conditions. As providers focus on delivering value-based and scalable care, accelerated adoption of nanorobotic technologies is observed across diverse healthcare settings worldwide.
Scope & Segmentation
- Type: Includes diagnostic nanorobots for real-time disease detection, surgical nanorobots optimized for minimally invasive interventions, and therapeutic nanorobots deployed in targeted drug delivery and repair mechanisms.
- Component: Key systems such as actuators, communication modules, power supplies, sensor networks, and embedded software platforms ensure autonomous operation and the accurate collection of clinical data.
- Mechanism of Action: Encompasses biological nanorobots modeled on cellular structures, hybrid designs integrating biological and synthetic components, and mechanical nanorobots engineered for specialized functions, offering flexibility across clinical use cases.
- Material: Utilizes carbon nanotubes, gold nanoparticles, graphene, lipid-based nanomaterials, magnetic nanoparticles, polymer nanoparticles, and quantum dots, each chosen to maximize biocompatibility and stability for sustained therapeutic impact.
- Application: Supports an array of medical needs, such as cancer therapeutics, enhanced imaging, precise site-specific drug administration, gene editing, internal physiological monitoring, regenerative medicine, and tissue reconstruction.
- End-Use: Adoption spans diagnostic centers, hospitals, clinics, pharmaceutical developers, and research organizations, each segmenting procurement strategies to address unique clinical challenges and operational preferences.
- Region: The Americas, Europe, Middle East & Africa, and Asia-Pacific are at the forefront of innovation, with regional policy initiatives, research infrastructure, and deployment frameworks contributing to evolving competitive dynamics.
Key Takeaways for Senior Decision-Makers
- Nanorobots support a new era of ultra-targeted therapies and cellular-level precision in patient care, which can streamline treatment pathways and potentially reduce recovery times.
- Strategic alliances between research institutions, manufacturers, and pharmaceutical enterprises facilitate the efficient advancement and upscaling of nanorobotic technologies from laboratory to clinical use.
- Emergent innovations in materials and software controls are enabling greater adaptability, compatibility, and longevity for devices within sensitive biomedical environments.
- Regional markets in North America, Europe, and Asia-Pacific are leading in regulatory harmonization and early-stage adoption, providing a blueprint for market entry and expansion strategies.
- Building intellectual property assets and leveraging specialized manufacturing centers allows organizations to foster sustainable differentiation and maintain continuous pipelines for innovation.
Tariff Impact on Supply and Cost Structures
Recent tariff impositions by the United States on imported nanorobot materials and components are reshaping supply chain resilience and cost management strategies. Manufacturers are reassessing sourcing protocols, diversifying supplier networks, and introducing operational safeguards to minimize financial exposure. Healthcare institutions are revisiting procurement models, favoring partnerships that enhance continuity and value-chain stability amid shifting trade environments.
Methodology & Data Sources
This assessment combines primary inputs from biomedical engineers, regulatory experts, and clinicians with secondary data from peer-reviewed journals, patent information, and policy analysis. By triangulating these sources, findings offer a holistic reflection of present-day technical, regulatory, and competitive factors within the nanorobotics landscape.
Why This Report Matters
- Provides objective benchmarking of innovation trends, helping senior leaders identify priority technology segments for strategic investment and market entry planning.
- Delivers actionable guidance on facilitating collaborations, refining intellectual property strategies, and identifying successful routes to commercialization.
- Enhances organizational planning with risk and resource analysis, focusing on supply chain shifts, regulatory requirements, and evolving reimbursement models that influence long-term opportunities.
Conclusion
As nanorobots redefine health technology frontiers, organizations equipped with specialized, data-driven insights from this market will be primed to deliver next-level medical care and innovation for the future.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Nanorobots in Healthcare market report include:- Bannari Amman Institute of Technology
- Carnegie Mellon University
- IBSA Foundation
- Karolinska Institutet
- Klocke Nanotechnik GmbH
- Koch Institute for Integrative Cancer Research
- Robeauté SAS
- Shenzhen Institute of Artificial Intelligence and Robotics for Society
- The Indian Institute of Science
- Theranautilus Private Limited
- UNC Eshelman School of Pharmacy
- University of Sydney
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 10.1 Billion |
| Forecasted Market Value ( USD | $ 38.39 Billion |
| Compound Annual Growth Rate | 20.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |