Global Adeno Associated Virus Vector Manufacturing Market - Key Trends & Drivers Summarized
Why Is AAV Vector Manufacturing Central to Gene Therapy Advancements?
AAV vector manufacturing is at the heart of revolutionary gene therapy advancements, serving as a critical tool for delivering therapeutic genes to target cells. The adeno associated virus, a non-pathogenic vector, has become the gold standard in gene delivery due to its high safety profile, low immunogenicity, and ability to efficiently transduce both dividing and non-dividing cells. These attributes make it ideal for treating a wide range of genetic disorders, including inherited retinal diseases, hemophilia, and neurological conditions.The increasing success of AAV-based gene therapies has fueled the demand for scalable, high-quality vector manufacturing processes. Biopharmaceutical companies and research organizations are investing heavily in developing robust manufacturing platforms to meet the growing needs of clinical trials and commercialized products. Furthermore, the versatility of AAV vectors in delivering genetic payloads has expanded their applications to emerging areas such as oncology and vaccines.
What Technological Innovations Are Shaping AAV Manufacturing?
The AAV vector manufacturing landscape is being transformed by technological advancements aimed at enhancing scalability, efficiency, and quality. High-capacity production platforms, such as suspension cell culture systems, are replacing traditional adherent systems, enabling large-scale manufacturing while reducing costs. Innovations in upstream and downstream processes, including optimized transfection techniques and high-efficiency purification methods, are improving overall vector yield and purity.The adoption of single-use bioreactors and automated systems is streamlining operations, minimizing contamination risks, and ensuring consistency across production batches. Advanced analytical tools are also playing a crucial role, allowing for precise characterization and quality control of AAV vectors. Furthermore, the integration of artificial intelligence (AI) and machine learning (ML) is enhancing process optimization, predictive maintenance, and troubleshooting, driving the efficiency of manufacturing workflows.
Who Are the Key End-Users Driving Market Demand?
The demand for AAV vector manufacturing spans a broad spectrum of stakeholders, including biopharmaceutical companies, academic research institutions, and contract development and manufacturing organizations (CDMOs). Biopharmaceutical firms are the primary drivers, leveraging AAV vectors to develop and commercialize gene therapies for a wide range of indications. These companies rely on robust manufacturing capabilities to ensure the consistent supply of vectors for clinical and commercial applications.Academic and research institutions are also significant contributors, as they explore innovative applications of AAV vectors in preclinical studies and early-stage clinical trials. CDMOs play a vital role in addressing capacity constraints faced by smaller companies and providing scalable manufacturing solutions. The rising prevalence of rare genetic disorders and the growing pipeline of AAV-based therapies have further expanded the customer base, with healthcare providers and regulatory agencies pushing for accelerated approvals and broader accessibility.
What Factors Are Driving Growth in the AAV Vector Manufacturing Market?
The growth in the adeno associated virus vector manufacturing market is driven by several factors, including the expanding pipeline of AAV-based gene therapies targeting a wide range of diseases. Technological advancements in production and purification methods are enhancing the scalability and efficiency of manufacturing processes, reducing costs and making therapies more accessible. The increasing prevalence of genetic disorders and the growing adoption of personalized medicine are also fueling demand for AAV vectors.End-use diversification, particularly in emerging areas such as oncology, vaccines, and rare disease treatments, is creating new opportunities for manufacturers. The rising investments from biopharmaceutical companies, coupled with government and private funding for gene therapy research, are accelerating innovation and production capacity expansion. Additionally, regulatory support for expedited pathways and initiatives promoting the development of advanced therapeutics are further driving the robust growth of the AAV vector manufacturing market.
Report Scope
The report analyzes the Adeno Associated Virus Vector Manufacturing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Scale of Operations (Clinical Scale of Operations, Preclinical Scale of Operations, Commercial Scale of Operations); Method (In Vitro Method, In Vivo Method); Therapeutic Area (Hematological Diseases Therapeutic Area, Infectious Diseases Therapeutic Area, Genetic Disorders Therapeutic Area, Neurological Disorders Therapeutic Area, Ophthalmic Disorders Therapeutic Area, Other Therapeutic Areas); Application (Cell Therapy Application, Gene Therapy Application, Vaccines Application).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Clinical Scale of Operations segment, which is expected to reach US$2.1 Billion by 2030 with a CAGR of a 22.8%. The Preclinical Scale of Operations segment is also set to grow at 18.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $289.5 Million in 2024, and China, forecasted to grow at an impressive 19.6% CAGR to reach $523.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Adeno Associated Virus Vector Manufacturing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Adeno Associated Virus Vector Manufacturing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Adeno Associated Virus Vector Manufacturing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3D Systems, Inc., Additive Industries B.V., EOS GmbH, Höganäs AB, Kennametal Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Adeno Associated Virus Vector Manufacturing market report include:
- 4D Molecular Therapeutics, Inc.
- Astellas Gene Therapies
- BioMarin Pharmaceutical, Inc.
- Charles River Laboratories International, Inc.
- Creative Biogene
- F. Hoffmann-La Roche Ltd.
- Oxford Biomedica PLC
- ProBio Inc.
- REGENXBIO, Inc.
- Sarepta Therapeutics, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 4D Molecular Therapeutics, Inc.
- Astellas Gene Therapies
- BioMarin Pharmaceutical, Inc.
- Charles River Laboratories International, Inc.
- Creative Biogene
- F. Hoffmann-La Roche Ltd.
- Oxford Biomedica PLC
- ProBio Inc.
- REGENXBIO, Inc.
- Sarepta Therapeutics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 205 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
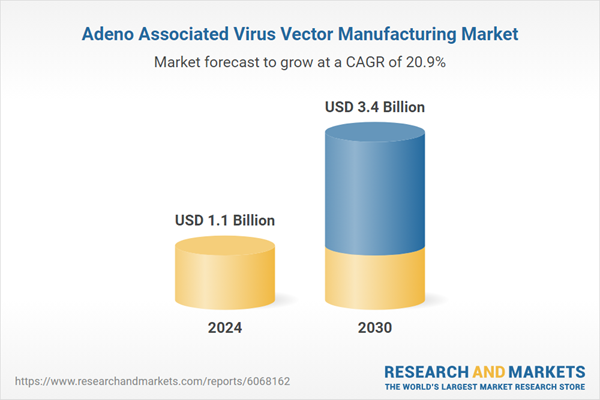
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 3.4 Billion |
| Compound Annual Growth Rate | 20.9% |
| Regions Covered | Global |