Global Multi-Cancer Early Detection Market - Key Trends & Drivers Summarized
How is Multi-Cancer Early Detection Reshaping Oncology Diagnostics?
The landscape of cancer detection is undergoing a seismic shift with the advent of Multi-Cancer Early Detection (MCED) technologies, a breakthrough that promises to revolutionize oncology diagnostics. Unlike conventional cancer screenings that target only a handful of malignancies - such as mammograms for breast cancer or colonoscopies for colorectal cancer - MCED tests utilize advanced genomic, epigenomic, and proteomic biomarkers to simultaneously detect multiple cancer types in a single assay. This is achieved through liquid biopsies, which analyze circulating tumor DNA (ctDNA), RNA signatures, and other molecular alterations in blood samples. As cancer remains a leading global cause of mortality, early detection is critical to improving survival rates. According to the World Health Organization (WHO), late-stage diagnoses account for a substantial proportion of cancer-related deaths, highlighting the urgent need for early, non-invasive, and comprehensive screening solutions. With artificial intelligence (AI) and machine learning (ML) algorithms enhancing biomarker identification, MCED tests are now more accurate, scalable, and accessible than ever before. Several biotech firms and research institutions have made significant strides in validating these tests through large-scale clinical trials, demonstrating their potential to identify cancers even before symptoms appear. The promise of reducing the burden of late-stage cancer diagnoses and improving patient outcomes has generated unprecedented excitement in the medical community, investors, and regulatory agencies alike.What Role Do Liquid Biopsies and AI Play in Advancing MCED?
At the core of MCED technology lies the integration of liquid biopsies and artificial intelligence, two game-changing innovations that have significantly elevated the accuracy and efficiency of multi-cancer screening. Traditional tissue biopsies, while effective, are often invasive, expensive, and limited to specific cancer types. Liquid biopsies, in contrast, offer a minimally invasive alternative that can detect a broad spectrum of cancers by identifying tumor-derived genetic fragments in the bloodstream. This technique has been particularly beneficial in detecting hard-to-screen cancers, such as pancreatic, ovarian, and esophageal malignancies, which often remain undiagnosed until advanced stages. AI-driven pattern recognition further amplifies the power of MCED by rapidly analyzing vast datasets of genetic, proteomic, and metabolic markers to differentiate between benign and malignant signals. AI models trained on millions of patient samples can refine diagnostic accuracy, minimize false positives and negatives, and even predict the tissue of origin for detected cancers. The growing adoption of AI in MCED has also enabled continuous learning, where algorithms evolve to recognize emerging cancer subtypes and adapt to new molecular discoveries. Notably, companies such as GRAIL, Guardant Health, and Exact Sciences have spearheaded efforts in commercializing AI-powered liquid biopsy tests, with extensive clinical validation backing their efficacy. Furthermore, regulatory approvals, such as the breakthrough designation granted by the U.S. Food and Drug Administration (FDA) for leading MCED tests, signal a paradigm shift in how cancer screening is approached. As these technologies mature, their integration into routine health check-ups is expected to become a new standard in preventive medicine, transforming early detection into a cost-effective, life-saving strategy.How Are Healthcare Policies and Market Investments Accelerating MCED Adoption?
Government policies, healthcare infrastructure, and investment trends play a pivotal role in shaping the trajectory of MCED market adoption. As healthcare systems worldwide grapple with the economic burden of late-stage cancer treatments, there is growing consensus on the cost-effectiveness of early detection strategies. Several national health agencies and insurance providers are exploring reimbursement models for MCED tests, acknowledging their potential to reduce overall cancer treatment costs while improving patient survival rates. In the United States, for instance, legislative efforts such as the Medicare Multi-Cancer Early Detection Screening Coverage Act aim to expand access to MCED diagnostics among high-risk populations. Similarly, the European Commission’ s Cancer Plan has prioritized early detection initiatives, pushing for greater integration of liquid biopsy-based screenings into national healthcare programs. Beyond policy-driven efforts, the influx of venture capital and private equity investments in the MCED sector has been instrumental in accelerating technological advancements and market expansion. According to industry reports, funding for precision oncology startups developing MCED solutions has surged dramatically over the past five years, with multi-billion-dollar valuations reflecting investor confidence in the technology’ s disruptive potential. Leading pharmaceutical giants have also entered strategic partnerships with biotech firms to co-develop and commercialize MCED products, further fueling research and innovation. However, despite these positive trends, challenges such as regulatory standardization, test affordability, and public awareness remain barriers to mass adoption. Addressing these hurdles through collaborative efforts between industry stakeholders, policymakers, and healthcare providers will be crucial in ensuring MCED becomes a widespread and equitable solution for early cancer detection.What Are the Key Market Drivers Shaping the Future of MCED?
The growth in the global Multi-Cancer Early Detection market is driven by several factors, including rapid technological advancements, increasing adoption of precision medicine, and the rising burden of cancer worldwide. Technological breakthroughs in next-generation sequencing (NGS) and digital PCR have significantly enhanced the sensitivity and specificity of MCED tests, making them more reliable for early-stage cancer detection. The integration of AI and big data analytics into diagnostic workflows has also streamlined biomarker discovery, allowing for faster and more accurate identification of cancer signatures. Additionally, the shift toward personalized medicine, where treatments and screenings are tailored to individual genetic profiles, has fueled demand for advanced MCED solutions. Consumer behavior is another critical driver, as awareness about the benefits of early cancer detection grows and patients increasingly seek proactive healthcare options. The rising prevalence of direct-to-consumer (DTC) genetic testing has also contributed to market expansion, with individuals opting for MCED tests as part of their personalized health monitoring strategies. From an end-use perspective, hospitals, diagnostic laboratories, and research institutions are among the key adopters of MCED technologies, integrating these tests into routine cancer screening programs. The expanding applications of MCED beyond oncology - such as in companion diagnostics and treatment monitoring - have further broadened market opportunities. Additionally, government initiatives aimed at reducing cancer mortality rates have driven policy frameworks that support MCED adoption. Emerging economies are also witnessing a surge in demand for advanced diagnostics, fueled by improving healthcare infrastructure and increasing disposable incomes. While market growth is promising, challenges such as regulatory complexities, ethical considerations surrounding genetic testing, and the need for large-scale validation studies must be addressed to sustain long-term adoption. Nevertheless, as innovation continues to push boundaries, MCED is poised to become a cornerstone in the future of cancer prevention and early intervention.Report Scope
The report analyzes the Multi Cancer Early Detection market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Gene Panel & LDT, Liquid Biopsy); End-Use (Hospitals End-Use, Diagnostic Laboratories End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Gene Panel & LDT segment, which is expected to reach US$1.4 Billion by 2030 with a CAGR of a 13.8%. The Liquid Biopsy segment is also set to grow at 18.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $296.0 Million in 2024, and China, forecasted to grow at an impressive 20.4% CAGR to reach $556.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Multi Cancer Early Detection Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Multi Cancer Early Detection Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Multi Cancer Early Detection Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agripolyane, Alpine Material Solutions, Ampacet Corporation, Barbier Groupe, BASF SE and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Multi Cancer Early Detection market report include:
- AnPac Bio-Medical Science Co., Ltd.
- Becton, Dickinson and Company (BD)
- Biocept, Inc.
- Burning Rock Biotech Limited
- Delfi Diagnostics, Inc.
- Epigenomics AG
- Exact Sciences Corporation
- Freenome Holdings, Inc.
- GRAIL, Inc.
- Guardant Health, Inc.
- Hologic, Inc.
- Illumina, Inc.
- Lucence Diagnostics Pte Ltd
- Natera, Inc.
- Oncimmune Holdings plc
- Prenetics Global Limited
- Qiagen N.V.
- Roche Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AnPac Bio-Medical Science Co., Ltd.
- Becton, Dickinson and Company (BD)
- Biocept, Inc.
- Burning Rock Biotech Limited
- Delfi Diagnostics, Inc.
- Epigenomics AG
- Exact Sciences Corporation
- Freenome Holdings, Inc.
- GRAIL, Inc.
- Guardant Health, Inc.
- Hologic, Inc.
- Illumina, Inc.
- Lucence Diagnostics Pte Ltd
- Natera, Inc.
- Oncimmune Holdings plc
- Prenetics Global Limited
- Qiagen N.V.
- Roche Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 214 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
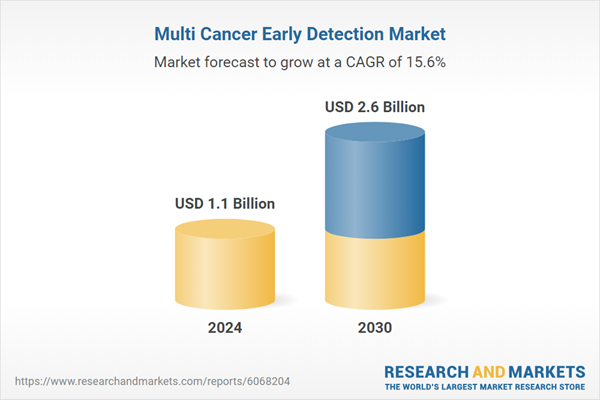
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 2.6 Billion |
| Compound Annual Growth Rate | 15.6% |
| Regions Covered | Global |