Global Omega-3 Prescription Drugs Market - Key Trends & Drivers Summarized
Why Are Omega-3 Prescription Drugs Gaining Clinical Ground in Cardiovascular and Metabolic Therapy?
Omega-3 prescription drugs are emerging as a crucial component in the pharmaceutical landscape, particularly for managing hypertriglyceridemia, cardiovascular disease (CVD), and other inflammation-driven chronic conditions. Unlike dietary supplements, these drugs are purified, concentrated, and clinically tested for efficacy and safety, offering higher dosages of eicosapentaenoic acid (EPA) and/or docosahexaenoic acid (DHA) suitable for medical intervention. Their role in reducing triglyceride levels, improving lipid profiles, and mitigating residual cardiovascular risk has been validated in large-scale clinical trials such as REDUCE-IT, which demonstrated cardiovascular outcome benefits from high-dose icosapent ethyl (EPA). These findings have driven inclusion of omega-3-based therapies in various treatment guidelines and have spurred a surge in prescriptions by cardiologists and primary care providers alike. The ability of these drugs to function alongside statins and other lipid-lowering agents enhances their value in managing complex dyslipidemia cases. Additionally, omega-3 prescription formulations have proven beneficial in conditions beyond heart health, including rheumatoid arthritis, chronic kidney disease, and cognitive decline, further expanding their therapeutic scope. With an aging population and a rising burden of chronic diseases worldwide, omega-3 prescription drugs are increasingly viewed not just as supplements, but as evidence-backed pharmaceuticals with a unique anti-inflammatory and cardioprotective mechanism of action.How Are Regulatory Approvals and Clinical Trials Expanding Therapeutic Applications?
Regulatory advancements and robust clinical research are significantly expanding the market potential for omega-3 prescription drugs. The FDA’ s approval of multiple omega-3-based formulations - such as icosapent ethyl and omega-3 acid ethyl esters - for severe hypertriglyceridemia has validated their therapeutic efficacy, opening pathways for broader clinical adoption. Meanwhile, ongoing trials are investigating their potential use in treating conditions like Alzheimer’ s disease, bipolar disorder, psoriasis, and certain cancers, fueled by omega-3s' known anti-inflammatory and cell membrane-stabilizing effects. In the EU and Asia-Pacific, regulatory agencies are also aligning with this trend, granting approvals and expanding reimbursement for high-purity EPA and DHA formulations. Pharmaceutical companies are increasingly investing in long-term studies to evaluate combination therapies and adjunctive uses of omega-3 drugs, particularly in statin-intolerant patients or those with persistent residual cardiovascular risk. Intellectual property strategies, such as extended-release technologies and novel lipid encapsulation systems, are being pursued to enhance bioavailability and minimize gastrointestinal side effects. These developments not only expand the therapeutic footprint of omega-3 prescription drugs but also create competitive differentiation in an otherwise crowded cardiovascular treatment space. As regulatory bodies demand more targeted and personalized treatment options, omega-3 drugs continue to gain traction for their dual role in both primary and secondary prevention of life-threatening conditions.Is Patient Demand for Natural and Safe Therapies Reshaping the Omega-3 Pharmaceutical Landscape?
Rising patient interest in natural, well-tolerated, and science-backed therapies is a significant force reshaping the omega-3 prescription drug market. As more consumers grow wary of long-term side effects from synthetic drugs - especially in managing chronic conditions - omega-3 therapies are being embraced for their favorable safety profiles and natural origins. This trend is particularly strong among older adults and patients with multiple comorbidities, who often require long-term pharmacological management and prefer gentler, non-toxic treatment options. Unlike over-the-counter fish oil supplements, prescription omega-3 drugs offer standardized dosages, controlled purity, and clinically validated outcomes, all of which increase patient and physician trust. The rise in health literacy and patient involvement in treatment decisions has created greater awareness of omega-3s' role in heart, brain, and joint health, further reinforcing their market relevance. Additionally, the shift toward preventive healthcare is positioning omega-3 prescription drugs as proactive, lifestyle-aligned tools for managing cardiovascular risk. Telemedicine platforms and e-pharmacy services are also contributing to easier access, broader outreach, and better medication adherence. Pharmaceutical companies are leveraging this growing patient awareness through direct-to-consumer campaigns, digital education, and personalized engagement strategies. As the perception of 'natural meets pharmaceutical-grade' continues to gain traction, omega-3 drugs are bridging the gap between traditional pharmacotherapy and wellness-centric, patient-preferred medicine.What’ s Fueling the Accelerated Growth of the Omega-3 Prescription Drugs Market Globally?
The growth in the omega-3 prescription drugs market is driven by several factors rooted in clinical innovation, chronic disease prevalence, regulatory momentum, and shifting healthcare preferences. One of the primary growth drivers is the global rise in cardiovascular and metabolic disorders - such as hyperlipidemia, diabetes, and obesity - which increase demand for adjunctive lipid-lowering therapies that go beyond statins. Additionally, favorable results from landmark studies like REDUCE-IT and JELIS have strengthened the clinical positioning of EPA-focused therapies, prompting widespread adoption in cardiology and internal medicine. The expanding elderly population - especially in North America, Europe, and Japan - is further contributing to demand, given the age-related increase in CVD, cognitive decline, and inflammation-related disorders. On the commercial side, pharmaceutical innovation in formulation technologies and dosage optimization has enhanced drug tolerability, bioavailability, and compliance, leading to improved therapeutic outcomes. Increasing regulatory approvals in emerging markets, coupled with rising insurance coverage and public health campaigns, are also facilitating market penetration. The blurring lines between nutraceuticals and pharmaceuticals have opened doors for pharma-nutrition collaborations and hybrid business models. Moreover, growing awareness of omega-3’ s broader benefits - ranging from eye and brain health to joint mobility - is prompting physicians to consider it for holistic treatment plans. Collectively, these dynamics are pushing omega-3 prescription drugs from a niche cardiovascular add-on to a mainstream, multifunctional therapeutic solution across global healthcare systems.Report Scope
The report analyzes the Omega 3 Prescription Drugs market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Drug (Vascepa Drugs, Lovaza Drugs, Other Drugs); Distribution Channel (Retail Pharmacies, Hospital Pharmacies, Online Pharmacies); Application (Cardiovascular Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Vascepa Drugs segment, which is expected to reach US$821.2 Million by 2030 with a CAGR of a 2.4%. The Lovaza Drugs segment is also set to grow at 3.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $356.8 Million in 2024, and China, forecasted to grow at an impressive 5.5% CAGR to reach $302.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Omega 3 Prescription Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Omega 3 Prescription Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Omega 3 Prescription Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Akzo Nobel N.V., Ashland Global Specialty Chemicals Inc., BASF SE, Croda International Plc, Ecogreen Oleochemicals Pte Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Omega 3 Prescription Drugs market report include:
- Abbott Laboratories
- Amarin Corporation plc
- Apotex Inc.
- AstraZeneca plc
- BASF SE
- Bayer AG
- Camber Pharmaceuticals, Inc.
- Dr. Reddy's Laboratories Ltd.
- GlaxoSmithKline plc (GSK)
- Grupo Ferrer Internacional, S.A.
- Hikma Pharmaceuticals PLC
- Mylan N.V.
- Natrapharm, Inc. (Patriot Pharmaceutical Corp.)
- Novartis International AG
- Pfizer Inc.
- Sandoz International GmbH
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Zydus Lifesciences Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Amarin Corporation plc
- Apotex Inc.
- AstraZeneca plc
- BASF SE
- Bayer AG
- Camber Pharmaceuticals, Inc.
- Dr. Reddy's Laboratories Ltd.
- GlaxoSmithKline plc (GSK)
- Grupo Ferrer Internacional, S.A.
- Hikma Pharmaceuticals PLC
- Mylan N.V.
- Natrapharm, Inc. (Patriot Pharmaceutical Corp.)
- Novartis International AG
- Pfizer Inc.
- Sandoz International GmbH
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Zydus Lifesciences Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 378 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
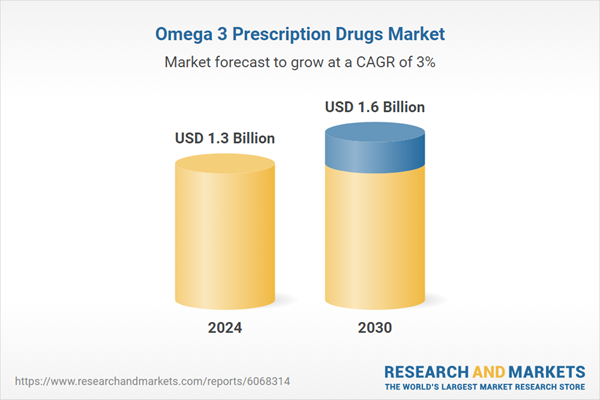
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 3.0% |
| Regions Covered | Global |