Pharmaceutical Manufacturing Equipment- Key Trends & Drivers Summarized
How Is Pharmaceutical Manufacturing Equipment Transforming Drug Production?
Pharmaceutical manufacturing equipment plays a pivotal role in ensuring the efficient, precise, and high-quality production of medications, including tablets, capsules, injectables, and biologics. As the industry embraces automation, process optimization, and stringent regulatory standards, advancements in manufacturing technology are reshaping drug production processes. Modern pharmaceutical equipment is designed to improve product consistency, enhance scalability, and reduce human error while maintaining compliance with Good Manufacturing Practices (GMP) and regulatory requirements set by agencies like the U.S. FDA, EMA, and WHO.A key aspect of pharmaceutical manufacturing is ensuring sterility and contamination control, particularly for aseptic and injectable drug production. High-speed filling lines, isolators, and clean-in-place (CIP) systems have become essential components of modern manufacturing plants. Additionally, continuous manufacturing (CM) is gaining traction over traditional batch processing, allowing for real-time monitoring and improved efficiency. The integration of Industry 4.0 technologies, such as the Internet of Things (IoT) and artificial intelligence (AI), is further enhancing process automation, predictive maintenance, and quality control, driving efficiency across the pharmaceutical supply chain.
What Market Trends Are Driving the Evolution of Pharmaceutical Manufacturing Equipment?
The pharmaceutical manufacturing equipment market is experiencing rapid transformation due to emerging trends that address the industry's evolving needs. One of the most significant trends is the rise of single-use technology (SUT), particularly in biologics manufacturing. Single-use bioreactors, filtration systems, and disposable tubing reduce contamination risks, enhance flexibility, and lower operational costs, making them an attractive alternative to traditional stainless-steel equipment. This shift is particularly beneficial for contract development and manufacturing organizations (CDMOs) and biotech firms focused on personalized medicine and cell and gene therapies.Another key trend is the growing adoption of continuous manufacturing (CM) over batch production. CM offers a more efficient and controlled approach to pharmaceutical production by enabling real-time quality assurance, reducing production time, and minimizing waste. Regulatory agencies, including the FDA, have been actively encouraging the adoption of CM to improve drug quality and supply chain resilience. In addition, 3D printing technology is revolutionizing pharmaceutical manufacturing by allowing for the production of personalized drug formulations and complex dosage forms, leading to improved patient outcomes.
Furthermore, the increasing demand for high-potency active pharmaceutical ingredients (HPAPIs) has driven advancements in containment and isolation technology. The need for specialized equipment, such as high-containment glove boxes and closed-system transfer devices (CSTDs), has surged as pharmaceutical manufacturers work with more potent compounds, including oncology drugs and hormone-based treatments. The focus on sustainability and energy-efficient equipment is also rising, with manufacturers investing in eco-friendly production systems that minimize carbon footprints and resource consumption.
How Are End-Use Applications Influencing the Demand for Pharmaceutical Equipment?
The demand for pharmaceutical manufacturing equipment is closely linked to the diverse applications across drug production segments, including oral solid dosage (OSD), parenteral drugs, biopharmaceuticals, and specialty medicines. The OSD segment, encompassing tablets and capsules, remains the largest consumer of manufacturing equipment, requiring advanced tablet presses, granulators, and coating machines to ensure precision and consistency. High-speed encapsulation machines are also in demand as manufacturers aim to enhance production efficiency while maintaining stringent quality control standards.In the injectable and biopharmaceutical segments, the demand for aseptic processing equipment is surging due to the growing production of biologics, vaccines, and gene therapies. This includes advanced fill-finish systems, lyophilization (freeze-drying) technology, and isolators that maintain sterility throughout the manufacturing process. The rise in personalized medicine and small-batch biologics has further increased the need for flexible, modular manufacturing equipment that can handle variable production scales.
Additionally, the pharmaceutical packaging sector is witnessing significant technological advancements. Smart packaging solutions, such as anti-counterfeiting technology and track-and-trace systems, are becoming integral to pharmaceutical manufacturing. Serialization and aggregation equipment are critical components in ensuring compliance with global regulations like the Drug Supply Chain Security Act (DSCSA) and the Falsified Medicines Directive (FMD), which mandate stringent tracking of pharmaceutical products throughout the supply chain.
What Factors Are Driving the Growth of the Pharmaceutical Manufacturing Equipment Market?
The growth in the pharmaceutical manufacturing equipment market is driven by several factors, including the increasing demand for automation, rising production of biologics, and evolving regulatory requirements. The industry’ s shift toward advanced manufacturing technologies, such as continuous processing, digital twin simulations, and robotics, is enhancing production efficiency and reducing operational costs. AI-powered predictive maintenance and real-time process analytics are further optimizing equipment utilization, minimizing downtime, and improving overall productivity.The expansion of biopharmaceutical production is another major growth driver, with companies investing heavily in state-of-the-art bioprocessing equipment to meet the rising demand for monoclonal antibodies, mRNA-based therapies, and personalized medicine. Additionally, the pharmaceutical industry’ s focus on sustainability and green manufacturing is driving investments in energy-efficient equipment and waste reduction technologies, aligning with global environmental standards.
Regulatory support for innovative manufacturing techniques, particularly continuous manufacturing and real-time quality control, is also accelerating market growth. Agencies like the FDA and EMA are encouraging pharmaceutical manufacturers to adopt advanced processing equipment that ensures drug quality and reduces production inefficiencies. Moreover, the expansion of CDMOs and contract manufacturing services is fueling demand for flexible and scalable production equipment that can handle diverse drug formulations and varying production volumes.
As the pharmaceutical industry continues to evolve, the demand for advanced manufacturing equipment will remain strong, shaping the future of drug production through automation, digitalization, and innovative processing technologies.
Report Scope
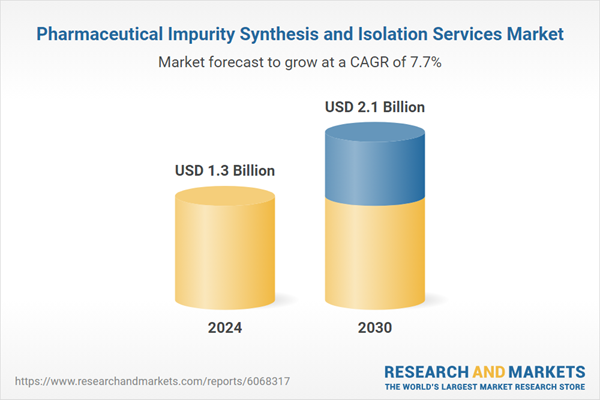
The report analyzes the Pharmaceutical Impurity Synthesis and Isolation Services market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Service (Isolation Services, Analytical Services, Synthesis Services); Impurity (Inorganic Impurities, Organic Impurities, Residual Solvents); Technique (Chromatography Technique, Spectroscopy Technique, Crystallization Technique, Hyphenated Technique, Other Techniques); Application (Commercial Manufacturing Application, Drug Development Application, Quality Control Application, Regulatory Compliance Application); End-Use (Biotech & Pharmaceutical Companies End-Use, Contract Research Organizations End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Isolation Services segment, which is expected to reach US$1.3 Billion by 2030 with a CAGR of a 8.9%. The Analytical Services segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $346.3 Million in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $328.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Impurity Synthesis and Isolation Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Impurity Synthesis and Isolation Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Impurity Synthesis and Isolation Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alpavit, ARMOR PROTEINES, BASF Corporation, Charotar Casein Company, Dastech International Inc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Pharmaceutical Impurity Synthesis and Isolation Services market report include:
- Alfa Chemistry
- Almac Group
- BOC Sciences
- Cambrex Corporation
- Catalent, Inc.
- Charles River Laboratories
- Epichem
- Eurofins Scientific
- Frontage Laboratories
- Intertek Group plc
- Laboratory Corporation of America Holdings (Labcorp)
- PCI Pharma Services
- Pharmaron
- Piramal Pharma Solutions
- SGS Société Générale de Surveillance SA
- Symeres
- Syngene International Limited
- Veeda Clinical Research
- Veeprho
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alfa Chemistry
- Almac Group
- BOC Sciences
- Cambrex Corporation
- Catalent, Inc.
- Charles River Laboratories
- Epichem
- Eurofins Scientific
- Frontage Laboratories
- Intertek Group plc
- Laboratory Corporation of America Holdings (Labcorp)
- PCI Pharma Services
- Pharmaron
- Piramal Pharma Solutions
- SGS Société Générale de Surveillance SA
- Symeres
- Syngene International Limited
- Veeda Clinical Research
- Veeprho
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 245 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 2.1 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |