Global Sterile Injectable Contract Manufacturing Market - Key Trends & Drivers Summarized
Why Is Contract Manufacturing of Sterile Injectables Becoming a Cornerstone of the Biopharma Supply Chain?
Sterile injectable contract manufacturing is increasingly central to the pharmaceutical and biotech industries, driven by the complexity, cost, and regulatory rigor associated with producing injectable drugs in sterile environments. As demand for injectable formulations rises - spanning biologics, vaccines, monoclonal antibodies, oncology drugs, and emergency care therapeutics - drug developers are outsourcing manufacturing to specialized CDMOs (Contract Development and Manufacturing Organizations) that offer the infrastructure, technical expertise, and compliance credentials necessary for sterile production. The need to maintain aseptic conditions, mitigate contamination risks, and adhere to stringent FDA, EMA, and WHO guidelines has made in-house production both risky and resource-intensive, especially for emerging biotech firms and specialty pharma companies. Moreover, sterile injectables often require high containment technologies, fill-finish services, and lyophilization capabilities that only a select group of contract manufacturers can deliver efficiently. As speed-to-market and production scalability become competitive differentiators in the post-pandemic era, outsourcing sterile injectables has become a strategic imperative, not just a cost-saving measure - especially in the race to commercialize life-saving therapies and respond to global health emergencies.How Are Technological Advancements and Regulatory Compliance Shaping Service Offerings?
The sterile injectable contract manufacturing market is being rapidly transformed by the adoption of advanced aseptic processing technologies, digitized manufacturing operations, and global harmonization of quality standards. State-of-the-art cleanroom designs, isolator and RABS (Restricted Access Barrier Systems) technology, and automated filling lines are enabling CDMOs to produce high-purity injectables with reduced contamination risks and improved batch consistency. Single-use technologies are increasingly being integrated into upstream and downstream workflows to minimize cross-contamination, reduce downtime, and increase flexibility - especially for multi-product facilities. Many contract manufacturers are investing in high-speed, multi-format filling lines that accommodate vials, prefilled syringes, ampoules, and cartridges, enabling greater versatility in handling customer demand. Digitalization through MES (Manufacturing Execution Systems), eBatch records, and real-time monitoring platforms is improving traceability, regulatory compliance, and audit readiness. CDMOs are also adapting to evolving global regulatory frameworks, achieving certifications such as cGMP, PIC/S, and ISO, which are increasingly required for cross-border commercialization. The ability to offer end-to-end solutions - including formulation, aseptic fill-finish, lyophilization, packaging, labeling, and serialization - is now a major value proposition for CDMOs looking to differentiate in a highly competitive and regulation-heavy environment.Where Is Demand Surging, and Which Therapeutic Areas Are Fueling Market Expansion?
The demand for sterile injectable contract manufacturing is surging across both developed and emerging markets, with North America and Europe leading in terms of CDMO capacity and innovation, while Asia-Pacific is rapidly gaining prominence due to cost efficiency and growing regulatory alignment. The U.S. remains the largest market, driven by a strong biotech ecosystem, advanced biologics development, and high outsourcing rates among small and mid-sized pharma companies. Europe follows closely, with countries like Germany, Switzerland, and Ireland hosting specialized facilities that serve global clientele. In Asia, India and China are expanding their sterile injectable infrastructure, drawing increased interest from multinational pharmaceutical companies seeking capacity, affordability, and regulatory-compliant production. Therapeutically, oncology leads the demand due to the prevalence of injectable chemotherapy and immunotherapy drugs. Infectious diseases, including vaccines and antibiotics, are also major growth drivers, especially in light of global preparedness for pandemics and antimicrobial resistance. Other high-demand segments include diabetes (injectable insulin and GLP-1 agonists), autoimmune diseases, and central nervous system disorders. Biologics and biosimilars, many of which require parenteral delivery, are further fueling demand, as drug pipelines become increasingly weighted toward complex molecules that require specialized sterile manufacturing solutions.What's Driving the Long-term Growth of the Sterile Injectable Contract Manufacturing Market Globally?
The growth in the sterile injectable contract manufacturing market is driven by a convergence of biopharma innovation, operational complexity, regulatory evolution, and global healthcare dynamics. A fundamental long-term driver is the proliferation of injectable biologics and advanced therapies, which require high-precision aseptic processes and highly controlled environments that are challenging to replicate in-house. As drug development pipelines shift toward niche and high-value therapeutics, companies are increasingly turning to CDMOs to access specialized capabilities without the burden of capital investment. Additionally, the growing prevalence of chronic and age-related diseases is fueling long-term demand for injectable treatments that offer rapid bioavailability and sustained efficacy. Regulatory authorities worldwide are mandating higher levels of sterility assurance, serialization, and data integrity - creating barriers to entry that CDMOs are uniquely positioned to overcome. Rising demand for rapid response capabilities, as seen during the COVID-19 pandemic, is pushing pharmaceutical companies to secure flexible, scalable partnerships for fill-finish and vaccine production. Furthermore, global drug shortages and the need for supply chain resilience are prompting companies to diversify manufacturing footprints, often through external partnerships. As the industry continues to prioritize speed, safety, and scalability in injectable therapeutics, sterile injectable contract manufacturing will remain a key strategic lever - supporting innovation, access, and sustainability in global drug development.Report Scope
The report analyzes the Sterile Injectable Contract Manufacturing market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Molecule Type (Small Molecule, Large Molecule); Therapeutic Area (Cancer, Diabetes, Cardiovascular Diseases, Central Nervous System Diseases, Infectious Disorders, Musculoskeletal, Anti-viral, Others); End-Use (Pharmaceutical Companies, Biopharmaceutical Companies, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecule segment, which is expected to reach US$16.9 Billion by 2030 with a CAGR of a 9.2%. The Large Molecule segment is also set to grow at 13.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.2 Billion in 2024, and China, forecasted to grow at an impressive 14.4% CAGR to reach $5.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sterile Injectable Contract Manufacturing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sterile Injectable Contract Manufacturing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sterile Injectable Contract Manufacturing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Accelerate Learning, ArithmeType, ASME (American Society of Mechanical Engineers), AT&T, Cambium Learning Group and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Sterile Injectable Contract Manufacturing market report include:
- AbbVie Contract Manufacturing
- Aenova Group
- Ajinomoto Bio-Pharma Services
- Alcami Corporation
- Baxter BioPharma Solutions
- Baxter International Inc.
- Bespak (Consort Medical)
- Boehringer Ingelheim
- Catalent, Inc.
- Cipla Inc.
- CordenPharma
- Delpharm
- Eurofins CDMO
- Famar Health Care Services
- Fresenius Kabi AG
- Grifols S.A.
- Hikma Pharmaceuticals plc
- Jubilant HollisterStier
- Lonza Group
- NextPharma Technologies
- Patheon (Thermo Fisher)
- Pfizer CentreOne
- Recipharm AB
- Samsung Biologics
- Sandoz International GmbH
- Siegfried Holding AG
- Thermo Fisher Scientific
- Unither Pharmaceuticals
- Vetter Pharma
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Contract Manufacturing
- Aenova Group
- Ajinomoto Bio-Pharma Services
- Alcami Corporation
- Baxter BioPharma Solutions
- Baxter International Inc.
- Bespak (Consort Medical)
- Boehringer Ingelheim
- Catalent, Inc.
- Cipla Inc.
- CordenPharma
- Delpharm
- Eurofins CDMO
- Famar Health Care Services
- Fresenius Kabi AG
- Grifols S.A.
- Hikma Pharmaceuticals plc
- Jubilant HollisterStier
- Lonza Group
- NextPharma Technologies
- Patheon (Thermo Fisher)
- Pfizer CentreOne
- Recipharm AB
- Samsung Biologics
- Sandoz International GmbH
- Siegfried Holding AG
- Thermo Fisher Scientific
- Unither Pharmaceuticals
- Vetter Pharma
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 387 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
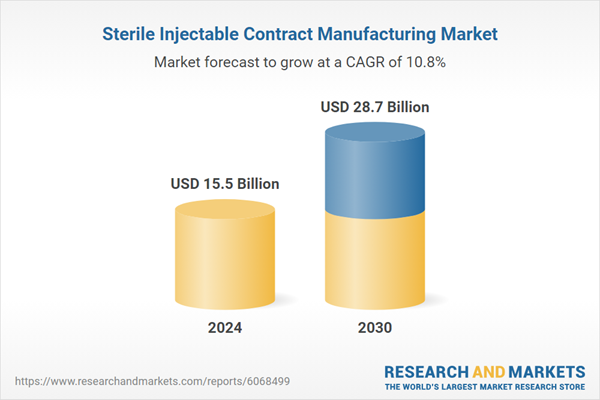
| Estimated Market Value ( USD | $ 15.5 Billion |
| Forecasted Market Value ( USD | $ 28.7 Billion |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |