Global Cosmetovigilance Market - Key Trends & Drivers Summarized
Why Is Cosmetovigilance Becoming Central to the Beauty Industry’ s Integrity?
Cosmetovigilance, the science of monitoring the safety of cosmetic products post-market, has evolved from a niche regulatory requirement into a critical pillar of brand trust and consumer safety. As the cosmetics industry expands rapidly - with thousands of new formulations and products introduced each year - the need to track, analyze, and respond to adverse reactions in real-world use has become more urgent. Unlike pharmaceuticals, cosmetics are used more broadly and frequently, often with complex layering of products, making safety surveillance challenging yet essential. Consumers today are more aware and vocal about skin sensitivities, allergic reactions, and product inconsistencies, prompting companies to implement robust cosmetovigilance systems to collect, manage, and analyze complaints or health-related incidents. Regulatory bodies, especially in regions like the EU under Regulation (EC) No 1223/2009, have made it mandatory for companies to maintain safety reports and submit serious undesirable effects (SUEs). This regulatory push, coupled with heightened consumer expectations, has turned cosmetovigilance into a competitive differentiator - companies that prioritize post-market safety and transparency are perceived as more credible and consumer-centric.Is Regulatory Expansion Driving Global Harmonization of Cosmetovigilance Practices?
Global regulatory alignment is emerging as a key catalyst for the growth of the cosmetovigilance market. While the European Union has long set the benchmark for cosmetic safety monitoring, other regions are steadily catching up with stricter guidelines and reporting frameworks. Countries like South Korea, Japan, Brazil, Canada, and India are introducing their own versions of post-market surveillance mandates, making it essential for global brands to establish standardized processes across multiple jurisdictions. This has led to a growing demand for centralized data management systems and harmonized reporting protocols that can support multilingual, multi-regional compliance. Additionally, collaborations between international regulatory bodies and industry groups are helping to define unified terminologies, risk thresholds, and response timelines, thus streamlining global cosmetovigilance efforts. The increasing scrutiny over ingredient safety - especially with rising concern around preservatives, fragrances, and nano-materials - is also prompting regulators to request more rigorous post-marketing surveillance data. As regulatory landscapes tighten worldwide, companies that invest early in scalable and compliant cosmetovigilance infrastructure stand to gain both operational resilience and reputational strength.How Are Technology and AI Revolutionizing Cosmetovigilance Capabilities?
Technological advancements are radically transforming how cosmetovigilance is conducted, making it faster, more accurate, and highly>What’ s Fueling the Growth of the Cosmetovigilance Market Across the Globe?
The growth in the cosmetovigilance market is driven by several factors related to technological integration, regulatory expansion, and evolving consumer expectations. Technologically, the adoption of AI-powered safety analytics, real-time reporting platforms, and automated data collection tools is making cosmetovigilance more scalable and efficient. From a regulatory standpoint, the proliferation of national and regional compliance frameworks is compelling companies to implement formalized surveillance processes, regardless of company size or product scope. The rise of clean beauty, sensitive skin products, and personalized cosmetics has intensified the need for post-market monitoring, as these formulations often include novel ingredients with limited long-term safety data. End-user behavior is also a major growth factor - consumers are more informed, more willing to report side effects, and increasingly expect full transparency around product safety. Social media and beauty influencer culture amplify adverse reactions quickly, pushing brands to act fast and communicate responsibly. Furthermore, cosmetic companies are recognizing the strategic value of cosmetovigilance in protecting brand reputation, maintaining consumer trust, and mitigating regulatory risk. The expansion of direct-to-consumer (D2C) models and cross-border e-commerce further necessitates robust safety monitoring systems, driving long-term investment and innovation in this critical area.Report Scope
The report analyzes the Cosmetovigilance market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase (Pre-clinical, Phase I, Phase II, Phase III, Phase IV); Services Type (Clinical Safety Testing Service, Document Writing Service, Risk Management Service, Case Intake Service, Case Triage Service, Data Entry & Acquisition Service, Tracking & Reporting Service); Category (Skincare Category, Makeup Category, Haircare Category, Perfume & Deodorants Category); Service Provider (In-house Service Provider, Contract Outsourcing Service Provider).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pre-clinical Phase segment, which is expected to reach US$5.7 Billion by 2030 with a CAGR of a 2.4%. The Phase I Phase segment is also set to grow at 2.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.0 Billion in 2024, and China, forecasted to grow at an impressive 5.5% CAGR to reach $2.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cosmetovigilance Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cosmetovigilance Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cosmetovigilance Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as All-Fill Inc., Bartelt Packaging LLC, BellatRx Inc., BW Packaging Systems, Coesia S.p.A. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Cosmetovigilance market report include:
- 4C Pharma Solutions
- Biologit
- Charles River Laboratories
- Cosmeservice
- Covance Inc.
- Delta PV
- Fortrea
- Freyr Solutions
- ICON plc
- Intertek Group plc
- IQVIA Holdings Inc.
- MartiFarm
- Medpace Holdings, Inc.
- Parexel International Corporation
- PharmaLex GmbH
- Piramal Pharma Solutions
- PRA Health Sciences
- Syneos Health
- Veeva Systems Inc.
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 4C Pharma Solutions
- Biologit
- Charles River Laboratories
- Cosmeservice
- Covance Inc.
- Delta PV
- Fortrea
- Freyr Solutions
- ICON plc
- Intertek Group plc
- IQVIA Holdings Inc.
- MartiFarm
- Medpace Holdings, Inc.
- Parexel International Corporation
- PharmaLex GmbH
- Piramal Pharma Solutions
- PRA Health Sciences
- Syneos Health
- Veeva Systems Inc.
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 483 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
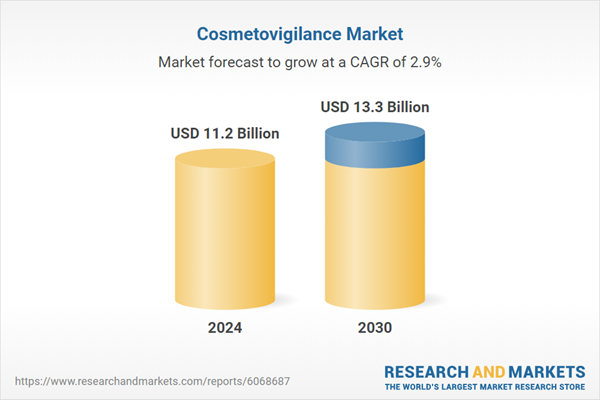
| Estimated Market Value ( USD | $ 11.2 Billion |
| Forecasted Market Value ( USD | $ 13.3 Billion |
| Compound Annual Growth Rate | 2.9% |
| Regions Covered | Global |