Global Laboratory Developed Tests Market - Key Trends & Drivers Summarized
Why Are Laboratory Developed Tests Transforming the Diagnostics Landscape?
Laboratory developed tests (LDTs) have become a cornerstone of modern diagnostic medicine, offering highly specialized and customizable testing solutions tailored to complex and rare conditions. Unlike commercial in vitro diagnostic (IVD) tests, which require regulatory approvals before reaching the market, LDTs are developed and validated within individual laboratories, allowing for rapid adaptation to emerging medical needs. This flexibility has made LDTs instrumental in personalized medicine, oncology, infectious disease detection, and genetic screening. As the demand for early and precise diagnostics continues to rise, LDTs are playing an increasingly vital role in bridging the gap between medical advancements and patient care.One of the key factors driving the adoption of LDTs is their ability to address niche diagnostic needs that may not be met by commercially available tests. In oncology, for instance, LDTs are widely used for tumor profiling, enabling oncologists to tailor treatment strategies based on genetic mutations specific to individual patients. The rapid advancement of genomic sequencing technologies has further strengthened the relevance of LDTs, allowing for the identification of hereditary conditions, pharmacogenomic responses, and rare genetic disorders. Additionally, the COVID-19 pandemic underscored the importance of LDTs in responding swiftly to novel pathogens, as many laboratories developed their own testing methodologies before commercial kits were widely available.
How Are Technological Innovations Enhancing the Capabilities of LDTs?
The evolution of laboratory developed tests has been closely linked to advancements in molecular biology, artificial intelligence (AI), and automation. Next-generation sequencing (NGS) and polymerase chain reaction (PCR)-based techniques have significantly improved the accuracy and speed of LDTs, enabling the detection of minute genetic variations associated with disease susceptibility and progression. AI-driven bioinformatics tools have further refined the analytical capabilities of LDTs, allowing for real-time data interpretation and pattern recognition in large genomic datasets. These technological breakthroughs have expanded the scope of LDT applications, from oncology and rare disease diagnostics to infectious disease surveillance and reproductive health.Automation has also played a pivotal role in the standardization and scalability of LDTs. High-throughput robotic systems now facilitate the processing of complex assays with minimal human intervention, reducing the risk of errors and increasing laboratory efficiency. The integration of cloud-based data storage and digital health platforms has enhanced accessibility, enabling healthcare providers to remotely access and analyze test results for more informed clinical decision-making. Moreover, advancements in liquid biopsy technology have paved the way for non-invasive LDTs, allowing for early cancer detection and monitoring through blood samples instead of traditional tissue biopsies. These innovations are driving greater adoption of LDTs across diverse healthcare settings, reinforcing their significance in precision medicine.
Are Regulatory Shifts Reshaping the Laboratory Developed Tests Market?
While LDTs have traditionally operated under a more flexible regulatory framework compared to commercially manufactured tests, recent regulatory shifts are poised to impact their development and adoption. In the United States, the Food and Drug Administration (FDA) has been considering increased oversight of LDTs to ensure test accuracy, reliability, and patient safety. This has sparked ongoing debates between regulatory agencies, diagnostic laboratories, and healthcare stakeholders regarding the balance between innovation and quality control. While stricter regulations may impose additional compliance burdens on laboratories, they also have the potential to enhance standardization and transparency, fostering greater trust in LDT-based diagnostics.Global regulatory landscapes are also evolving, with countries like the European Union implementing the In Vitro Diagnostic Regulation (IVDR) framework, which affects LDTs and imposes more rigorous validation requirements. These changes have prompted many laboratories to invest in quality management systems, accreditation processes, and clinical validation studies to align with evolving regulatory standards. Despite these challenges, the demand for LDTs remains strong, particularly in regions where regulatory flexibility allows for rapid adaptation to emerging medical conditions. As regulatory agencies refine their approach to LDT oversight, laboratories will need to navigate compliance requirements while maintaining their ability to innovate and deliver high-value diagnostic solutions.
What Are the Key Growth Drivers Fueling the Laboratory Developed Tests Market?
The growth in the laboratory developed tests market is driven by several factors, including the increasing prevalence of complex diseases, advancements in diagnostic technologies, rising demand for personalized medicine, and evolving healthcare policies. One of the primary drivers is the growing burden of cancer, cardiovascular diseases, and genetic disorders, which require highly specific and sensitive diagnostic tests. LDTs offer the flexibility to develop targeted assays for early disease detection, treatment monitoring, and risk assessment, making them invaluable in precision medicine initiatives. As healthcare systems shift toward proactive and preventive care models, the demand for LDTs continues to rise.Another major factor driving market growth is the expansion of genomic and molecular testing. The declining cost of genomic sequencing has made advanced LDTs more accessible, allowing for widespread adoption in clinical and research settings. The rise of companion diagnostics, which help determine the most effective treatments for individual patients, has further fueled LDT adoption in oncology and pharmacogenomics. Additionally, the increasing integration of AI and machine learning into laboratory workflows has improved the efficiency and accuracy of LDTs, driving confidence among healthcare providers and patients.
The growing emphasis on decentralized and point-of-care testing has also contributed to the expansion of LDTs, particularly in response to the COVID-19 pandemic. Many diagnostic laboratories developed their own rapid testing solutions to meet urgent public health demands, highlighting the agility of LDTs in crisis situations. Furthermore, the expansion of telemedicine and digital health platforms has increased the demand for remote and home-based diagnostic solutions, where LDTs play a crucial role in providing timely and accessible testing options.
Despite regulatory uncertainties, the laboratory developed tests market is expected to experience sustained growth as demand for specialized diagnostics continues to rise. The ongoing investments in precision medicine, coupled with advancements in biomarker discovery and high-throughput sequencing, will further drive innovation in LDTs. As laboratories navigate the evolving regulatory landscape, collaborations between research institutions, healthcare providers, and technology companies will be key in ensuring that LDTs remain a critical component of modern diagnostic medicine.
Report Scope
The report analyzes the Laboratory Developed Tests market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Technology (Immunoassays Technology, Hematology & Coagulation Technology, Molecular Diagnostics Technology, Microbiology Technology, Clinical Chemistry Technology, Histology / Cytology Technology, Flow Cytometry Technology, Mass Spectroscopy Technology, Other Technologies); Application (Oncology Application, Genetic Disorders / Inherited Disease Application, Infectious & Parasitic Diseases Application, Immunology Application, Endocrine Application, Nutritional & Metabolic Disease Application, Cardiology Application, Mental / Behavioral Disorder Application, Pediatrics-specific Testing Application, Hematology / General Blood Testing Application, Bodily Fluid Analysis Application, Toxicology Application, Other Diseases Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Immunoassays Technology segment, which is expected to reach US$5.1 Billion by 2030 with a CAGR of a 7.7%. The Hematology & Coagulation Technology segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.5 Billion in 2024, and China, forecasted to grow at an impressive 9.7% CAGR to reach $3.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Laboratory Developed Tests Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Laboratory Developed Tests Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Laboratory Developed Tests Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alphatec Spine, Cook Medical, DePuy Synthes (Johnson & Johnson), G-21 S.r.l., Globus Medical and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Laboratory Developed Tests market report include:
- 23andMe, Inc.
- Abbott
- Agilent Technologies Inc.
- ARUP Laboratories
- Bio-Rad Laboratories, Inc.
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Guardant Health
- Illumina, Inc.
- Invitae Corporation
- Labcorp
- Mayo Clinic Laboratories
- Myriad Genetics, Inc.
- Natera, Inc.
- NeoGenomics Laboratories
- OPKO Health, Inc.
- QIAGEN
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Sonic Healthcare
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 23andMe, Inc.
- Abbott
- Agilent Technologies Inc.
- ARUP Laboratories
- Bio-Rad Laboratories, Inc.
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Guardant Health
- Illumina, Inc.
- Invitae Corporation
- Labcorp
- Mayo Clinic Laboratories
- Myriad Genetics, Inc.
- Natera, Inc.
- NeoGenomics Laboratories
- OPKO Health, Inc.
- QIAGEN
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Sonic Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 317 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
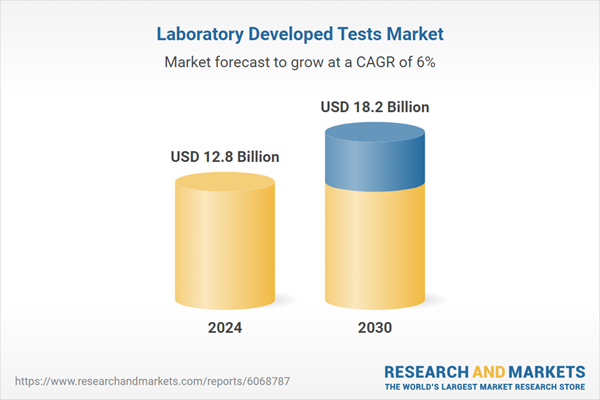
| Estimated Market Value ( USD | $ 12.8 Billion |
| Forecasted Market Value ( USD | $ 18.2 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |