Global Monkeypox Testing Market - Key Trends & Drivers Summarized
Why Has the Demand for Monkeypox Testing Surged in Recent Years?
The global outbreak of monkeypox has underscored the critical need for rapid and reliable diagnostic testing to contain the spread of the virus. Monkeypox, a viral zoonotic disease caused by the monkeypox virus, presents symptoms similar to smallpox, including fever, skin lesions, and swollen lymph nodes. With cases rising worldwide, governments and healthcare institutions have ramped up testing efforts to identify infections early and implement isolation measures. The demand for diagnostic testing has increased due to the highly transmissible nature of the virus, especially in urban and high-density population areas. Additionally, the emergence of asymptomatic and mild cases has highlighted the necessity for sensitive molecular tests that can accurately detect infections even in the absence of visible symptoms. The expansion of global travel and interconnected economies has further accelerated the spread of monkeypox, driving the urgent need for widespread testing.What Technological Innovations Are Enhancing Monkeypox Testing Capabilities?
The development of advanced diagnostic techniques has played a crucial role in improving the accuracy and speed of monkeypox testing. Molecular-based methods such as real-time polymerase chain reaction (RT-PCR) have become the gold standard for detecting monkeypox due to their high sensitivity and specificity. Rapid antigen tests are also being developed to provide point-of-care solutions for quick screening in high-risk populations. Additionally, next-generation sequencing (NGS) is being utilized to track viral mutations and understand transmission patterns. The integration of artificial intelligence (AI) in diagnostic tools has further improved the efficiency of test result interpretation, reducing the workload on healthcare professionals. At-home testing kits are also being explored to increase accessibility and encourage self-monitoring in vulnerable communities.How Are Government Regulations and Public Health Initiatives Influencing the Market?
Governments and health organizations worldwide have implemented extensive testing strategies to control monkeypox outbreaks, fueling demand for diagnostic solutions. The World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), and other global health agencies have issued guidelines on testing protocols, pushing for increased investment in laboratory infrastructure. Governments have also fast-tracked regulatory approvals for novel testing kits to accelerate deployment in healthcare settings. Public health campaigns emphasizing early detection and self-isolation have contributed to the rising demand for affordable and widely available testing options. The expansion of mobile testing units and community-based screening programs has further bolstered market growth.What Are the Key Factors Driving Growth in the Monkeypox Testing Market?
The growth in the monkeypox testing market is driven by several factors, including increased global outbreaks, advancements in diagnostic technologies, and government-backed testing initiatives. One of the primary drivers is the rising investment in infectious disease surveillance, which has led to the expansion of diagnostic laboratories and rapid testing infrastructure. The increased adoption of PCR-based testing, coupled with the development of portable and rapid antigen tests, is further fueling market expansion. Additionally, growing awareness about monkeypox and its potential long-term health impacts has encouraged widespread testing, particularly in high-risk populations. The ongoing research into serological and at-home testing solutions is expected to create new opportunities in the market. As healthcare systems continue to prioritize early detection and containment, the monkeypox testing market is poised for significant growth in the coming years.Report Scope
The report analyzes the Monkeypox Testing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Technology (Polymerase Chain Reaction Technology, Lateral Flow Assay Technology, Other Technologies); End-Use (Diagnostic Laboratories End-Use, Hospitals & Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Polymerase Chain Reaction Technology segment, which is expected to reach US$83.3 Million by 2030 with a CAGR of a 4.2%. The Lateral Flow Assay Technology segment is also set to grow at 2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $33.3 Million in 2024, and China, forecasted to grow at an impressive 6.3% CAGR to reach $29.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Monkeypox Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Monkeypox Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Monkeypox Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Anderson Advanced Ingredients, Apura Ingredients, Archer Daniels Midland Company, Cargill, Incorporated, Cumberland Packing Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Monkeypox Testing market report include:
- Aegis Sciences Corporation
- Applied DNA Clinical Labs, LLC
- ARUP Laboratories
- BayCare Laboratories, LLC
- Baylor Scott & White Advanced Diagnostics Laboratory
- BioReference Health, LLC
- CDR Laboratories, Inc
- Cepheid
- Curative Labs
- DevLab Bio
- Fulgent Genetics
- Houston Methodist Hospital Pathology
- Integrity Laboratories, LLC
- Ipsum Diagnostics LLC
- Johns Hopkins Medical Microbiology Laboratory
- Labcorp
- Mayo Clinic Laboratories
- Quest Diagnostics
- Sonic Healthcare
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aegis Sciences Corporation
- Applied DNA Clinical Labs, LLC
- ARUP Laboratories
- BayCare Laboratories, LLC
- Baylor Scott & White Advanced Diagnostics Laboratory
- BioReference Health, LLC
- CDR Laboratories, Inc
- Cepheid
- Curative Labs
- DevLab Bio
- Fulgent Genetics
- Houston Methodist Hospital Pathology
- Integrity Laboratories, LLC
- Ipsum Diagnostics LLC
- Johns Hopkins Medical Microbiology Laboratory
- Labcorp
- Mayo Clinic Laboratories
- Quest Diagnostics
- Sonic Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 279 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
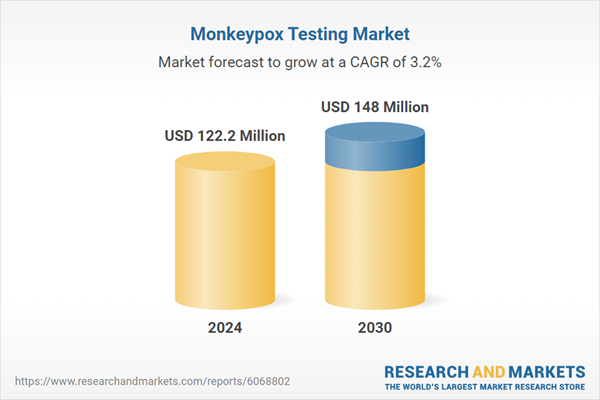
| Estimated Market Value ( USD | $ 122.2 Million |
| Forecasted Market Value ( USD | $ 148 Million |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |