Global Pharmaceutical Cleaning Validation Market - Key Trends & Drivers Summarized
What Is Pharmaceutical Cleaning Validation and Why Is It Crucial for the Industry?
Pharmaceutical cleaning validation refers to the process of ensuring that pharmaceutical manufacturing equipment is cleaned to a level that meets regulatory standards and does not pose a risk of contamination or affect product quality. This process is crucial for maintaining product safety, ensuring compliance with Good Manufacturing Practices (GMP), and preventing cross-contamination between batches or different products. Cleaning validation involves verifying that cleaning procedures are effective and repeatable, including the removal of residues, active pharmaceutical ingredients (APIs), detergents, and other contaminants from equipment surfaces.Cleaning validation is particularly vital in the pharmaceutical industry due to the potential impact that contamination can have on patient safety. Any residue left on equipment from previous products can cause adverse reactions, impact the efficacy of the drug, or pose health risks. Additionally, the pharmaceutical industry is highly regulated, and regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national authorities require stringent cleaning validation procedures. This process not only ensures that products are safe but also helps manufacturers maintain their credibility and avoid costly product recalls, fines, and other penalties.
Why Is the Pharmaceutical Cleaning Validation Market Expanding?
The pharmaceutical cleaning validation market is expanding due to several factors, including the increasing regulatory pressure on pharmaceutical manufacturers, the growing complexity of drug formulations, and the rising emphasis on quality and safety in the pharmaceutical industry. One of the primary drivers of market growth is the increasing regulatory requirements around cleaning processes. As regulatory bodies continue to impose stricter guidelines for pharmaceutical manufacturing and quality assurance, companies are investing more in cleaning validation to ensure compliance. These regulations require evidence that cleaning procedures are both effective and reproducible, making cleaning validation an essential part of the pharmaceutical production process.The growing complexity of drug formulations and the rise of combination products also contribute to the market's expansion. As pharmaceutical companies develop more complex formulations, such as biologics, gene therapies, and combination drugs, the risk of contamination increases. These products often require highly specialized and sterile manufacturing environments. Cleaning validation ensures that equipment used for different products, especially those with different formulations or therapeutic purposes, does not cross-contaminate. The need to validate cleaning processes in response to these increasingly complex formulations is driving greater demand for cleaning validation services and technologies.
Moreover, the increasing focus on quality and safety in the pharmaceutical industry is another key factor contributing to the market's growth. Pharmaceutical companies are prioritizing product safety and quality to meet both consumer expectations and regulatory standards. Ensuring that manufacturing equipment is properly cleaned and free from contaminants is a critical part of maintaining high-quality standards. The need to avoid potential safety risks, reduce operational costs, and prevent costly recalls is prompting more pharmaceutical companies to implement and invest in comprehensive cleaning validation procedures.
What Key Trends Are Shaping the Future of Pharmaceutical Cleaning Validation?
Several key trends are shaping the future of the pharmaceutical cleaning validation market, including the increasing adoption of automation and digitalization, the rise of more stringent regulatory guidelines, and the growing focus on sustainable cleaning practices. One of the most significant trends is the increasing use of automation and digital technologies in cleaning validation processes. Automation allows pharmaceutical companies to streamline and standardize cleaning procedures, reducing human error and ensuring more consistent and reproducible results. Digital technologies such as data analytics, sensors, and real-time monitoring systems are being integrated into cleaning validation practices to enhance efficiency, accuracy, and traceability. These advancements enable manufacturers to validate cleaning processes more quickly and effectively while maintaining compliance with regulatory requirements.The rise of more stringent regulatory guidelines is another key trend that is shaping the future of cleaning validation. Regulatory bodies are continuously updating and tightening cleaning validation standards to ensure product safety and quality. For instance, regulators are increasingly emphasizing the need for risk-based approaches to cleaning validation, taking into account factors such as the potential for cross-contamination, the nature of the drugs being produced, and the manufacturing processes used. Pharmaceutical manufacturers are adapting to these stricter regulations by investing in more robust cleaning validation systems and practices, ensuring that their operations remain compliant and meet the highest standards of quality and safety.
Sustainability is also becoming an important focus in the pharmaceutical cleaning validation market. As environmental concerns grow, the pharmaceutical industry is shifting towards more sustainable cleaning practices. This includes the use of environmentally friendly cleaning agents, reduced water and energy consumption, and more efficient waste management during the cleaning process. Manufacturers are looking for ways to reduce the environmental impact of their operations while maintaining high standards of cleanliness and safety. The growing demand for green cleaning solutions is driving the development of new technologies and practices that align with sustainability goals without compromising the effectiveness of the cleaning process.
What Are the Key Drivers of Growth in the Pharmaceutical Cleaning Validation Market?
The growth in the pharmaceutical cleaning validation market is driven by several factors, including increasing regulatory pressure, the growing complexity of pharmaceutical manufacturing processes, and the rising demand for high-quality and safe drug products. One of the key drivers is the increasing regulatory pressure on pharmaceutical companies to maintain rigorous cleaning validation processes. As regulatory agencies such as the FDA, EMA, and others continue to impose strict requirements for drug manufacturing, cleaning validation has become a critical component of compliance. Pharmaceutical companies must ensure that all equipment is properly cleaned to prevent cross-contamination, maintain product quality, and meet safety standards. These regulatory requirements are prompting companies to invest more in cleaning validation technologies, services, and expertise.The growing complexity of drug manufacturing processes is another significant driver for the market. The rise of biologics, combination drugs, and more complex formulations means that cleaning validation is becoming more challenging. With the increased risk of cross-contamination between different products, especially those with varying compositions and active ingredients, pharmaceutical companies need to implement more advanced and tailored cleaning validation procedures. This has led to an increased demand for specialized cleaning validation services that can handle the complexities of modern pharmaceutical manufacturing.
The rising demand for high-quality and safe pharmaceutical products is also contributing to the growth of the cleaning validation market. As patients and healthcare providers demand higher-quality products, pharmaceutical companies are prioritizing product safety and efficacy. Cleaning validation is a key part of this focus, ensuring that medications are produced without contamination and that all equipment used in production is properly sanitized. Furthermore, the increased awareness of patient safety and quality control has led pharmaceutical companies to place greater emphasis on maintaining the highest standards in their manufacturing processes, driving growth in the cleaning validation sector.
Lastly, advancements in cleaning validation technologies are enabling companies to implement more effective and efficient cleaning processes. Innovations such as automated systems, real-time monitoring, and advanced data analytics are improving the accuracy and speed of cleaning validation procedures. As these technologies become more accessible and affordable, they are helping pharmaceutical companies streamline their operations, reduce costs, and maintain compliance with ever-evolving regulatory requirements. The continued development of new and improved cleaning validation solutions is a key factor driving growth in the market.
Report Scope
The report analyzes the Pharmaceutical Cleaning Validation market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Products (Small Molecule Drug, Peptides, Proteins, Cleaning Detergent); Validation Test (Non-specific Tests, Product-specific Analytical Tests).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecule Drug segment, which is expected to reach US$9.3 Billion by 2030 with a CAGR of a 3.9%. The Peptides segment is also set to grow at 6.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $5.1 Billion in 2024, and China, forecasted to grow at an impressive 8.3% CAGR to reach $5.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Cleaning Validation Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Cleaning Validation Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Cleaning Validation Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alconox Inc., Almac Group, Ash Stevens, Charles River Laboratories International, Inc., Compliance Team LLC and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Pharmaceutical Cleaning Validation market report include:
- Alconox Inc.
- Almac Group
- Ash Stevens
- Charles River Laboratories International, Inc.
- Compliance Team LLC
- CoreRx, Inc
- Dalton Pharma Services
- Ecolab
- Eurofins Scientific
- GMP Insiders
- Intertek Group plc
- Nelson Laboratories, LLC
- PharmOutsourcing
- Pritchard Industries
- QbD Group
- Reading Scientific Services Ltd (RSSL)
- SGS SA
- STERIS plc
- Suez Water Technologies & Solutions
- Veolia Water Technologies
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alconox Inc.
- Almac Group
- Ash Stevens
- Charles River Laboratories International, Inc.
- Compliance Team LLC
- CoreRx, Inc
- Dalton Pharma Services
- Ecolab
- Eurofins Scientific
- GMP Insiders
- Intertek Group plc
- Nelson Laboratories, LLC
- PharmOutsourcing
- Pritchard Industries
- QbD Group
- Reading Scientific Services Ltd (RSSL)
- SGS SA
- STERIS plc
- Suez Water Technologies & Solutions
- Veolia Water Technologies
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 271 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
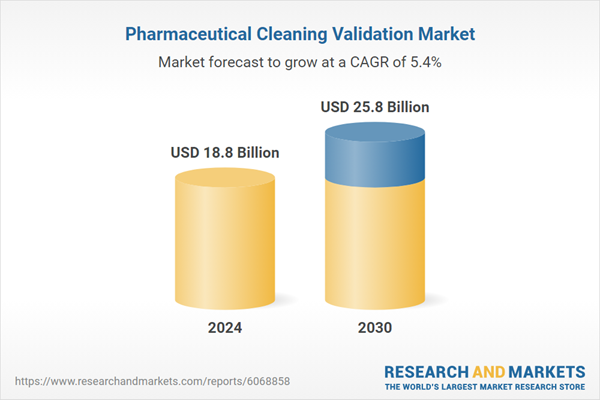
| Estimated Market Value ( USD | $ 18.8 Billion |
| Forecasted Market Value ( USD | $ 25.8 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |