Global Regulatory Information Management System Market - Key Trends & Drivers Summarized
Why Is Regulatory Information Management Becoming Essential for Compliance?
Regulatory information management systems (RIMS) have become crucial for industries that require efficient tracking, submission, and management of regulatory data. Pharmaceutical, life sciences, chemical, and financial sectors rely on these systems to ensure compliance with complex global regulatory requirements. RIMS platforms enable centralized data management, reducing compliance risks and improving regulatory submission efficiency. As governments tighten regulations and increase transparency requirements, businesses are investing in RIMS solutions to maintain compliance, avoid penalties, and streamline regulatory reporting. With the increasing digitalization of regulatory processes, RIMS has become an indispensable tool for managing regulatory operations effectively.How Are Technological Innovations Enhancing Regulatory Information Management?
The adoption of cloud-based RIMS solutions and AI-driven analytics is revolutionizing regulatory information management by improving data accuracy, accessibility, and compliance tracking. AI-powered automation is reducing manual workload by generating regulatory reports, ensuring faster approvals, and minimizing errors. Blockchain integration is also enhancing data security and traceability, providing a tamper-proof system for managing compliance records. Additionally, predictive analytics is enabling companies to anticipate regulatory changes and proactively adjust compliance strategies. These innovations are making RIMS platforms more robust, scalable, and adaptable to evolving regulatory landscapes.What Market Trends Are Driving the Adoption of RIMS?
The increasing adoption of digital transformation strategies in compliance management has fueled demand for RIMS across industries. Life sciences and pharmaceutical companies are leveraging RIMS for efficient regulatory submissions and lifecycle management of drugs and medical devices. Additionally, regulatory agencies worldwide are encouraging electronic submissions and standardized data formats, further driving the need for advanced RIMS solutions. The integration of RIMS with enterprise resource planning (ERP) and customer relationship management (CRM) platforms is enhancing workflow automation and regulatory intelligence. As companies prioritize transparency and efficiency in compliance, RIMS adoption is expected to continue rising.What Are the Key Growth Drivers of the RIMS Market?
The growth in the global regulatory information management system market is driven by increasing regulatory requirements, advancements in AI-powered compliance solutions, and the expansion of cloud-based regulatory management platforms. The pharmaceutical and life sciences industries' need for real-time regulatory data tracking has fueled demand for RIMS solutions. Additionally, the growing emphasis on global regulatory harmonization is encouraging companies to implement standardized compliance management systems. The rise of automation, data analytics, and blockchain in regulatory processes is further supporting market expansion. As industries seek to optimize compliance operations, the RIMS market is expected to witness sustained growth, driven by continuous technological innovation.Report Scope
The report analyzes the Regulatory Information Management Systems market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vertical (Pharmaceutical Sector, Medical Device Sector, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 48 companies featured in this Regulatory Information Management Systems market report include -
- Accenture
- Amplexor
- ArisGlobal
- Bioclinica
- Dassault Systèmes
- DDi (Document Databases International)
- Deloitte
- DXC Technology
- Generis Knowledge Management
- IQVIA
- Kalypso
- Lockpath (a NAVEX Global Company)
- LORENZ Life Sciences Group
- MasterControl
- Medidata Solutions
- Oracle Corporation
- PAREXEL International
- Parexel International Corporation
- Phlexglobal
- PSC Software
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pharmaceutical Vertical segment, which is expected to reach US$2.2 Billion by 2030 with a CAGR of a 9.5%. The Medical Device Vertical segment is also set to grow at 7.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $589.4 Million in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $732.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Regulatory Information Management Systems Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Regulatory Information Management Systems Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Regulatory Information Management Systems Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACA Group, Accell Clinical Research, LLC, Andersen, Bentley Biomedical Consulting, Capgemini SE and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 48 Featured):
- Accenture
- Amplexor
- ArisGlobal
- Bioclinica
- Dassault Systèmes
- DDi (Document Databases International)
- Deloitte
- DXC Technology
- Generis Knowledge Management
- IQVIA
- Kalypso
- Lockpath (a NAVEX Global Company)
- LORENZ Life Sciences Group
- MasterControl
- Medidata Solutions
- Oracle Corporation
- PAREXEL International
- Parexel International Corporation
- Phlexglobal
- PSC Software
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Accenture
- Amplexor
- ArisGlobal
- Bioclinica
- Dassault Systèmes
- DDi (Document Databases International)
- Deloitte
- DXC Technology
- Generis Knowledge Management
- IQVIA
- Kalypso
- Lockpath (a NAVEX Global Company)
- LORENZ Life Sciences Group
- MasterControl
- Medidata Solutions
- Oracle Corporation
- PAREXEL International
- Parexel International Corporation
- Phlexglobal
- PSC Software
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
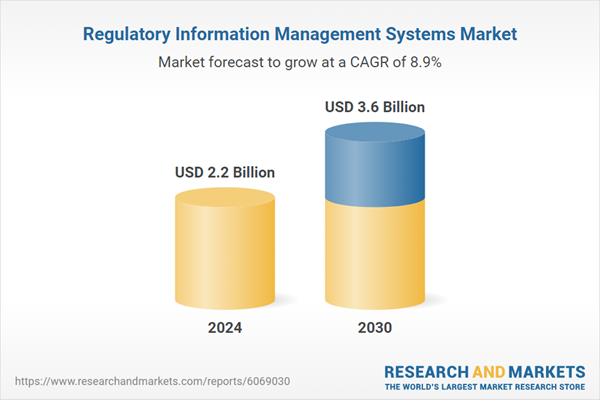
| Estimated Market Value ( USD | $ 2.2 Billion |
| Forecasted Market Value ( USD | $ 3.6 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |