Global Minimal Residual Disease Testing Market - Key Trends & Drivers Summarized
What Is Minimal Residual Disease Testing and Why Is It Important?
Minimal Residual Disease (MRD) testing refers to highly sensitive testing methods used to detect very small amounts of cancerous cells remaining in a patient’ s body after treatment, which are not detectable by standard diagnostic tools. MRD testing is critical in determining the effectiveness of cancer treatment, predicting the risk of relapse, and guiding subsequent therapy decisions. It is particularly important in hematologic cancers such as leukemia, lymphoma, and multiple myeloma, where microscopic amounts of abnormal cells can persist in the body even after clinical remission. By identifying these remaining cells, MRD testing provides clinicians with valuable information that can help tailor treatment plans, monitor disease recurrence, and improve patient outcomes.Techniques used for MRD testing include flow cytometry, polymerase chain reaction (PCR), and next-generation sequencing (NGS). These methods allow for the detection of abnormalities in blood or bone marrow samples at a much higher sensitivity level than traditional imaging or blood tests. For instance, PCR can identify specific genetic markers associated with certain cancers, while NGS can uncover mutations or genetic alterations in a broader range of cancer cells. The ability to detect these residual cells with high precision has revolutionized the management of hematologic malignancies, offering both a tool for early intervention and a way to gauge the success of ongoing treatments.
Why Is the Minimal Residual Disease Testing Market Growing?
The global market for MRD testing is expanding rapidly due to several key factors, including increasing cancer incidence, advancements in testing technology, and a greater emphasis on personalized medicine. The rising global burden of cancer, particularly hematologic cancers such as leukemia and lymphoma, has spurred demand for more accurate diagnostic and monitoring tools. As healthcare systems aim to improve survival rates and treatment outcomes, MRD testing has become an essential part of cancer management. The ability to detect cancer at its earliest stages, even when it is not visible through conventional imaging techniques, is a game-changer for clinicians and patients alike.Moreover, the growing awareness of the benefits of early relapse detection and the role of MRD testing in personalized treatment strategies is further driving the market. Personalized medicine focuses on tailoring treatment to the specific characteristics of each patient’ s disease, including genetic factors and how the disease responds to various therapies. MRD testing plays a pivotal role in this approach, as it helps clinicians adjust treatment plans in real-time, ensuring that patients receive the most effective therapies and reducing unnecessary treatments. These advancements in personalized care, coupled with a rise in patient demand for more effective and precise cancer treatments, are accelerating the adoption of MRD testing worldwide.
What Key Trends Are Shaping the Future of MRD Testing?
The future of MRD testing is being shaped by several transformative trends, notably technological advancements, regulatory developments, and a shift toward liquid biopsy techniques. One of the most significant trends is the continuous improvement of sensitivity and accuracy in MRD detection methods. Next-generation sequencing (NGS) and digital PCR, for example, are becoming more refined and are now able to detect MRD at levels as low as one cancer cell in a million, significantly improving the ability to monitor minimal residual disease over time. These innovations are enhancing early relapse detection and allowing clinicians to make more informed decisions about whether to adjust treatment plans, such as introducing additional chemotherapy or bone marrow transplants.Another key trend is the increasing popularity of liquid biopsy techniques. Traditionally, MRD testing required invasive procedures such as bone marrow aspirates. However, liquid biopsy, which involves testing blood samples, is becoming more widely used due to its less invasive nature, convenience, and ability to provide real-time results. This approach is especially attractive in monitoring patients who have completed initial treatment or those in remission, as it can be used for frequent testing without the need for repeated biopsies. Liquid biopsies are also paving the way for non-invasive MRD testing in other cancers beyond hematologic malignancies, expanding the scope of MRD applications in oncology.
What Are the Key Drivers of Growth in the MRD Testing Market?
The growth in the minimal residual disease testing market is driven by several factors, including advancements in molecular biology, an increasing focus on cancer surveillance, and the rising adoption of precision medicine. As our understanding of cancer biology deepens, so does our ability to develop sophisticated testing methods that provide more sensitive and reliable results. The growth of molecular techniques, such as next-generation sequencing and PCR, has made it possible to detect minute amounts of cancer cells in the bloodstream or bone marrow, allowing for more accurate monitoring of disease progression and remission.The shift toward precision medicine is another key driver behind the increased demand for MRD testing. With personalized treatments becoming more prevalent, MRD testing provides a valuable tool for assessing the effectiveness of therapies and predicting patient outcomes. By identifying residual disease before relapse occurs, MRD testing enables clinicians to make timely interventions and modify treatment plans to optimize outcomes. Furthermore, the growing prevalence of hematologic cancers, coupled with an increasing number of patients seeking better management options, is driving demand for MRD testing services. Healthcare providers, particularly in oncology centers, are increasingly integrating MRD testing into routine cancer care, making it an integral part of long-term cancer management strategies.
Finally, regulatory approvals and reimbursement support are also playing a significant role in market growth. The FDA and other regulatory bodies have started to approve MRD testing as part of routine clinical trials for cancer drugs, boosting the integration of MRD testing in both clinical research and practice. In addition, more insurance companies are covering MRD testing, further encouraging widespread adoption. As these factors continue to align, the MRD testing market is expected to continue its upward trajectory, driving innovation in cancer care and offering patients better chances of long-term survival and quality of life.
Report Scope
The report analyzes the Minimal Residual Disease Testing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Technology (Flow Cytometry Technology, Polymerase Chain Reaction Technology, Next Generation Sequencing Technology, Other Technologies); Cancer Type (Hematological Malignancy, Solid Tumors); End-Use (Hospitals & Specialty Clinics, Diagnostic Laboratories, Academic & Research Institutes, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Flow Cytometry Technology segment, which is expected to reach US$2.1 Billion by 2030 with a CAGR of a 13%. The Polymerase Chain Reaction Technology segment is also set to grow at 11.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $621.1 Million in 2024, and China, forecasted to grow at an impressive 15.8% CAGR to reach $925.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Minimal Residual Disease Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Minimal Residual Disease Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Minimal Residual Disease Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Minimal Residual Disease Testing market report include:
- Adaptive Biotechnologies Corporation
- ArcherDX, Inc.
- ARUP Laboratories Inc.
- Asuragen Inc.
- Biocept, Inc.
- Bio-Rad Laboratories, Inc.
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd.
- Guardant Health
- Invitae Corporation
- Invivoscribe Inc.
- Labcorp Inc.
- Laboratory Corporation of America Holdings
- Natera Inc.
- NeoGenomics Laboratories, Inc.
- OncoOne
- QIAGEN N.V.
- Quest Diagnostics
- Sysmex Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Adaptive Biotechnologies Corporation
- ArcherDX, Inc.
- ARUP Laboratories Inc.
- Asuragen Inc.
- Biocept, Inc.
- Bio-Rad Laboratories, Inc.
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd.
- Guardant Health
- Invitae Corporation
- Invivoscribe Inc.
- Labcorp Inc.
- Laboratory Corporation of America Holdings
- Natera Inc.
- NeoGenomics Laboratories, Inc.
- OncoOne
- QIAGEN N.V.
- Quest Diagnostics
- Sysmex Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 383 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
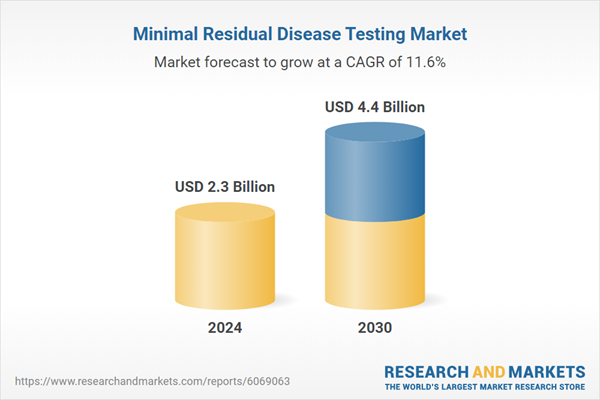
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 4.4 Billion |
| Compound Annual Growth Rate | 11.6% |
| Regions Covered | Global |