Global NUT Midline Carcinoma Treatment Market - Key Trends & Drivers Summarized
Why Is NUT Midline Carcinoma Driving an Urgent Rethink in Rare Cancer Therapeutics?
NUT midline carcinoma (NMC) is an exceptionally rare and aggressive form of cancer, characterized by chromosomal rearrangements involving the NUTM1 gene, typically resulting in the BRD4-NUT fusion. The disease most commonly affects midline structures of the head, neck, and thorax, and has a devastatingly poor prognosis, with median survival often measured in months rather than years. Due to its rarity and rapid progression, NMC has long been underdiagnosed or misclassified, limiting both patient access to effective treatment and broader pharmaceutical investment. However, the increasing ability to identify NMC through molecular diagnostics and next-generation sequencing (NGS) is changing the landscape. With awareness rising among oncologists and pathologists, more cases are being correctly diagnosed earlier, creating an emerging but urgent patient population in need of targeted therapies. This has prompted renewed interest from drug developers and research institutions focused on rare and ultra-rare cancers. As the oncology community becomes more focused on precision medicine and genetic drivers of cancer, NMC is gaining recognition as a test case for the development of novel, biomarker-guided treatments. This shift is accelerating demand for targeted therapeutic options, as well as supportive diagnostics, companion biomarkers, and clinical trial networks specialized in treating aggressive, genomically defined malignancies.How Are Targeted Therapies and Novel Approaches Redefining the Treatment Outlook for NMC?
While traditional treatment regimens for NUT midline carcinoma - typically involving surgery, radiation, and chemotherapy - have shown limited success, the advent of targeted therapeutics is opening new doors for improved outcomes. The most promising area of innovation lies in BET (bromodomain and extraterminal domain) inhibitors, which specifically target the BRD4-NUT fusion protein that drives the disease’ s aggressive growth. Several BET inhibitors are currently under clinical investigation, showing potential to inhibit tumor proliferation and delay disease progression in NMC patients. Parallel research is exploring histone deacetylase (HDAC) inhibitors, immune checkpoint inhibitors, and even experimental CRISPR-based approaches to disrupt oncogenic fusion activity. These novel treatments are frequently being tested in basket trials that group patients by genetic mutations rather than tumor location, offering a more flexible and focused clinical trial environment for NMC. Additionally, advancements in liquid biopsy, ctDNA tracking, and personalized oncology platforms are allowing for real-time monitoring of treatment efficacy and resistance patterns. Pediatric and young adult populations - where NMC is disproportionately diagnosed - are also benefiting from research in age-specific pharmacodynamics and dosing strategies. As regulatory agencies become more receptive to accelerated pathways for rare cancer therapies, drug developers are investing in orphan drug designations, expanded access programs, and adaptive trial designs to bring effective NMC treatments to market faster. These efforts are gradually building a more hopeful treatment framework for what has long been one of the most therapeutically underserved cancer types.What Structural and Clinical Challenges Are Shaping the NMC Therapeutics Landscape?
Despite growing interest, the market for NUT midline carcinoma treatment remains fraught with complex challenges, both clinical and structural. Foremost among them is the ultra-low incidence rate, which makes patient recruitment for trials difficult and limits the commercial incentive for drug development. NMC often presents with nonspecific symptoms and shares histological features with other poorly differentiated carcinomas, making accurate diagnosis highly dependent on specialized molecular testing - resources not always available in community oncology settings. There is also a lack of standardized treatment protocols and consensus guidelines, leaving oncologists reliant on case reports, institutional experience, or extrapolated data from other cancers. The highly aggressive nature of NMC further compounds these issues, as patients often progress rapidly, limiting the window for intervention and clinical trial enrollment. Geographic disparities in access to molecular diagnostics and experimental therapies mean that many patients remain undiagnosed or undertreated. From a regulatory standpoint, the ultra-orphan status of NMC necessitates a different economic and ethical model for drug development, with reimbursement structures and public-private partnerships playing a critical role. Additionally, emotional and psychological support for patients and caregivers is underdeveloped due to the disease’ s rarity and grim prognosis. However, increasing collaboration between academic centers, rare cancer alliances, and pharmaceutical companies is beginning to address these systemic gaps, offering a more coordinated approach to data sharing, patient advocacy, and international trial design.What’ s Driving the Growth of the NUT Midline Carcinoma Treatment Market?
The growth in the NUT midline carcinoma treatment market is driven by several interrelated factors grounded in technological progress, evolving clinical strategies, and institutional commitment to rare disease innovation. First, advancements in genomic profiling, especially next-generation sequencing and immunohistochemistry, are leading to more accurate and earlier diagnoses of NMC, thereby expanding the identifiable patient pool. Second, the clinical pipeline for BET inhibitors, HDAC inhibitors, and epigenetic modulators is maturing, with promising candidates entering late-stage trials and gaining regulatory attention through orphan drug and breakthrough therapy designations. Third, a paradigm shift in oncology drug development - centered on biomarker-driven and histology-agnostic therapies - is supporting the inclusion of NMC patients in broader precision medicine initiatives. Fourth, global investment in rare disease research, supported by venture capital, government grants, and philanthropic funding, is incentivizing CDMOs, biotechs, and big pharma alike to develop treatments tailored to ultra-rare malignancies like NMC. Fifth, the expansion of decentralized and digital clinical trial models is easing logistical barriers and facilitating patient participation in geographically dispersed regions. Finally, growing awareness among clinicians, supported by medical education campaigns and advocacy groups, is leading to more proactive case identification and referral to centers of excellence. Together, these forces are laying the foundation for a more structured, innovative, and hopeful treatment landscape for NUT midline carcinoma - transforming a previously overlooked condition into a critical focus area within rare oncology.Report Scope
The report analyzes the NUT Midline Carcinoma Treatment market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Treatment (Chemotherapy Treatment, Targeted Therapy Treatment, Immunotherapy Treatment, Radiation Therapy Treatment, Other Treatments); Route of Administration (Intravenous Route of Administration, Oral Route of Administration, Other Route of Administrations); End-Use (Hospitals End-Use, Specialty Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Chemotherapy Treatment segment, which is expected to reach US$14.6 Billion by 2030 with a CAGR of a 9.3%. The Targeted Therapy Treatment segment is also set to grow at 8.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $5.8 Billion in 2024, and China, forecasted to grow at an impressive 14% CAGR to reach $7.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global NUT Midline Carcinoma Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global NUT Midline Carcinoma Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global NUT Midline Carcinoma Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M Company, B. Braun Melsungen AG, Baxter International Inc., Cardinal Health, Inc., Coloplast A/S and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this NUT Midline Carcinoma Treatment market report include:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BeiGene Ltd.
- Boehringer Ingelheim GmbH
- Bristol-Myers Squibb Company
- C4 Therapeutics, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- GSK plc
- Incyte Corporation
- Ipsen Biopharmaceuticals, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis International AG
- Pfizer Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Zenas BioPharma
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BeiGene Ltd.
- Boehringer Ingelheim GmbH
- Bristol-Myers Squibb Company
- C4 Therapeutics, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- GSK plc
- Incyte Corporation
- Ipsen Biopharmaceuticals, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis International AG
- Pfizer Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Zenas BioPharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 383 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
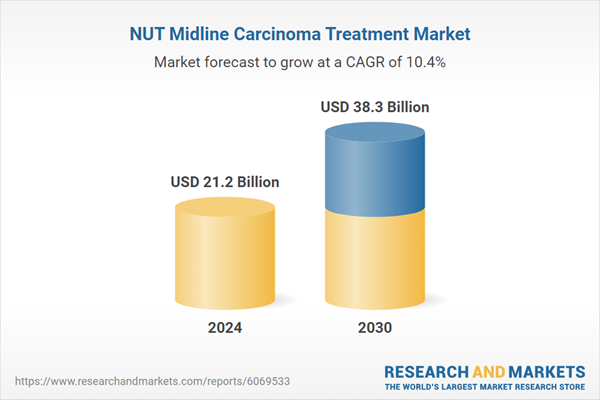
| Estimated Market Value ( USD | $ 21.2 Billion |
| Forecasted Market Value ( USD | $ 38.3 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |