Population Screening Market - Key Trends & Drivers Summarized
What Is Population Screening and Why Is It Crucial for Public Health?
Population screening is a proactive approach in public health designed to identify diseases, genetic conditions, or risk factors in asymptomatic individuals at an early stage. Unlike diagnostic tests, which confirm a condition in individuals with symptoms, population screening aims to detect health concerns before symptoms develop, allowing for early intervention, improved treatment outcomes, and reduced healthcare costs. Common examples include cancer screening (mammograms, colonoscopies, and HPV tests), newborn screening for metabolic and genetic disorders, cardiovascular risk screening, and infectious disease screening (HIV, hepatitis, and tuberculosis tests).Governments and healthcare organizations worldwide implement screening programs to reduce disease burden and mortality rates. Mass screening programs have historically been effective in reducing cervical cancer through Pap smears, improving cardiovascular health through lipid profiling, and preventing hereditary conditions through genetic carrier screening. With the increasing prevalence of chronic diseases and age-related conditions, healthcare systems are expanding population screening efforts to cover more conditions, integrate new technologies, and personalize risk assessment based on demographic, genetic, and lifestyle factors.
How Is Technology Revolutionizing Population Screening Methods?
Advancements in medical technology, artificial intelligence (AI), and big data analytics are significantly improving the accuracy, efficiency, and scalability of population screening programs. One of the most transformative developments is the use of AI-powered imaging and machine learning algorithms in radiology and pathology. AI can analyze mammograms, lung scans, and retinal images with higher accuracy than traditional methods, reducing false positives and enabling earlier detection of cancer, diabetic retinopathy, and neurodegenerative diseases.Another major breakthrough is the rise of liquid biopsy and multi-cancer early detection (MCED) tests, which use blood samples to detect circulating tumor DNA (ctDNA) and other biomarkers indicative of early-stage cancers. These non-invasive tests offer a safer and more convenient alternative to traditional tissue biopsies, making them ideal for large-scale screening programs. Additionally, advancements in genomics and whole-genome sequencing (WGS) have made it possible to screen individuals for inherited diseases, pharmacogenomic responses, and predisposition to complex conditions like Alzheimer's and diabetes.
The integration of wearable health technology and digital biomarkers is also reshaping how screening is conducted. Devices such as smartwatches, continuous glucose monitors, and ECG-equipped wearables allow for continuous health monitoring, real-time data collection, and early detection of arrhythmias, hypertension, and metabolic disorders. These innovations enable a shift from one-time screening tests to ongoing health surveillance, allowing healthcare providers to intervene at the earliest signs of abnormal physiological changes.
What Are the Key Trends Shaping the Expansion of Population Screening?
One of the most significant trends in population screening is the move toward personalized and risk-based screening protocols. Traditionally, screening guidelines were based on age and broad population criteria, but emerging research in precision medicine and risk stratification is leading to tailored screening recommendations. For example, instead of universal breast cancer screening starting at age 40, genetic risk assessment and family history analysis now help determine whether an individual should start earlier or undergo more frequent screenings. This approach minimizes unnecessary tests while improving outcomes for high-risk individuals.The expansion of home-based and self-administered screening tests is also driving a shift in healthcare accessibility. The COVID-19 pandemic accelerated the adoption of at-home diagnostic tests, telemedicine consultations, and mail-in sample collection kits, which have since been expanded to HPV, colorectal cancer, and infectious disease screening. These decentralized models allow for higher participation rates, reduced healthcare disparities, and increased convenience, especially for populations in rural areas or those with limited access to medical facilities.
Another growing trend is the integration of population screening into workplace wellness programs and public-private partnerships. Large corporations and insurance providers are now offering preventive health screenings as part of employee benefits, recognizing that early disease detection reduces long-term medical costs and improves workforce productivity. Similarly, public health agencies are collaborating with private biotech firms to develop cost-effective, AI-powered screening solutions that can be deployed at scale. These partnerships help expand access to screening initiatives and optimize public health outcomes.
What Factors Are Driving the Growth of the Population Screening Market?
The growth in the population screening market is driven by several factors, including technological advancements, increased government funding, expanding disease coverage, and the rising prevalence of non-communicable diseases (NCDs). The increasing global burden of chronic diseases such as cancer, cardiovascular disease, and diabetes has heightened the need for proactive disease management through early detection, prompting governments and healthcare organizations to prioritize screening initiatives and allocate larger budgets for preventive care.Another key driver is the falling costs of genetic sequencing and biomarker analysis, making advanced screening technologies more affordable and accessible. Next-generation sequencing (NGS) and AI-powered diagnostics are now being integrated into routine screening programs, allowing for early identification of genetic predispositions, cancer mutations, and autoimmune markers. This has led to higher adoption rates of genetic and precision screening techniques, particularly in developed healthcare markets such as the United States, Europe, and Japan.
Regulatory initiatives and policy reforms are also fueling the expansion of organized screening programs. Governments worldwide are implementing mandates for cancer screening coverage, newborn screening expansion, and infectious disease testing, ensuring that a larger percentage of the population benefits from early detection services. Additionally, advances in cloud computing and AI-driven data analytics are improving electronic health records (EHR) integration, allowing for better tracking of patient screening histories, risk factors, and follow-up interventions.
Furthermore, the role of consumer-driven healthcare and digital health platforms is reshaping how individuals participate in screening programs. The rise of direct-to-consumer (DTC) health testing companies has empowered individuals to take control of their health by purchasing at-home screening kits and genetic risk assessments without requiring physician referrals. As consumer awareness about preventive healthcare and longevity-focused diagnostics grows, the demand for convenient, technology-enabled screening solutions is expected to continue accelerating worldwide.
Report Scope
The report analyzes the Population Screening market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Region Type (National Screening, State-wise Screening); Product Type (Hardware Equipment, Testing / Lab Services, Analytics / Interpretation); Screening Type (Mass Screening, Premium Screening); Business Type (Hospitals, Research Institutes, Diagnostic Labs).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the National Screening segment, which is expected to reach US$25.7 Billion by 2030 with a CAGR of a 5.1%. The State-wise Screening segment is also set to grow at 2.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $7.4 Billion in 2024, and China, forecasted to grow at an impressive 8.2% CAGR to reach $7.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Population Screening Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Population Screening Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Population Screening Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alibaba Bursting Boba Suppliers, BobaVida, BossenStore.com, Bursting Boba, Empire Eagle Food Co., Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Population Screening market report include:
- Abbott Laboratories
- Aduro
- Agilent Technologies
- ARUP Laboratories
- CHC Wellbeing
- eHealthScreenings
- Empower Health Services
- F. Hoffmann-La Roche Ltd.
- Gene by Gene, Ltd.
- Health Advocate
- Health Designs
- Illumina, Inc.
- LGC Limited
- Novogene Corporation
- Onsite Health Diagnostics
- QIAGEN N.V.
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- US Wellness
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Aduro
- Agilent Technologies
- ARUP Laboratories
- CHC Wellbeing
- eHealthScreenings
- Empower Health Services
- F. Hoffmann-La Roche Ltd.
- Gene by Gene, Ltd.
- Health Advocate
- Health Designs
- Illumina, Inc.
- LGC Limited
- Novogene Corporation
- Onsite Health Diagnostics
- QIAGEN N.V.
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- US Wellness
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 467 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
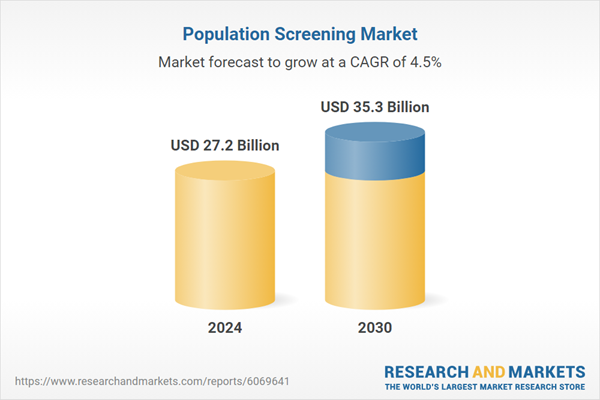
| Estimated Market Value ( USD | $ 27.2 Billion |
| Forecasted Market Value ( USD | $ 35.3 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |