Immunoassay Interference Blocker: What’ s Making This Solution Critical for Accurate Diagnostics?
Global Immunoassay Interference Blocker Market - Key Trends & Drivers Summarized
The global market for immunoassay interference blockers is growing steadily as the demand for high-precision diagnostics surges across clinical, research, and point-of-care (POC) settings. Immunoassays - used widely to detect hormones, proteins, pathogens, and drugs - are vulnerable to interference from endogenous substances such as heterophilic antibodies, rheumatoid factors, human anti-animal antibodies (HAAAs), or complement proteins. These interferences can distort assay results, leading to false positives or negatives, which in turn risk misdiagnosis, inappropriate treatments, and increased healthcare costs. Immunoassay interference blockers are specialized reagents or buffers designed to neutralize such interfering substances, ensuring the specificity, reliability, and reproducibility of test results.As healthcare systems globally pivot toward early disease detection and personalized treatment, the accuracy of diagnostic assays has become paramount. This shift is particularly evident in oncology, endocrinology, infectious disease, and therapeutic drug monitoring - where diagnostic errors due to interference can have critical implications. With rapid diagnostic testing and high-throughput immunoassay platforms now commonplace in clinical labs, there is a parallel need to integrate high-performance interference blockers into assay workflows. As a result, both diagnostic manufacturers and research institutions are increasingly investing in anti-interference technologies to enhance the robustness and clinical utility of immunoassays.
What Are the Technological Advances Transforming Interference Mitigation in Immunoassays?
Advancements in immunoassay formulation and interference blocker design are transforming how laboratories achieve analytical precision. Manufacturers are now developing proprietary blocker formulations that target specific classes of interfering substances - such as human anti-mouse antibodies (HAMA), biotin, or autoimmune factors - without compromising antigen-antibody binding kinetics. These blockers are incorporated at various stages of assay development: in assay buffers, sample diluents, and wash solutions. Cutting-edge blockers utilize recombinant proteins, monoclonal antibodies, or synthetic peptides to selectively neutralize interferents while preserving analyte signal integrity.Moreover, the emergence of multiplex assays and highly sensitive platforms - such as chemiluminescence, electrochemiluminescence, and digital ELISA - demands more sophisticated interference mitigation. To meet these needs, companies are embedding interference detection and compensation algorithms into assay software, complemented by engineered blockers that dynamically adjust to sample matrix variability. Additionally, AI-based assay design and high-throughput screening techniques are being used to test and optimize blocker efficacy against a broad range of biological interferences. These technologies not only ensure higher diagnostic confidence but also reduce the need for confirmatory testing, thus enhancing workflow efficiency.
Where Is the Demand for Immunoassay Interference Blockers Growing - And What’ s Driving It?
Demand for immunoassay interference blockers is strongest in North America and Europe, where regulatory compliance, rigorous quality standards, and advanced diagnostic infrastructure drive high assay reliability expectations. The U.S., in particular, leads the market, driven by extensive clinical lab testing, FDA mandates for diagnostic accuracy, and continuous innovation from IVD (in vitro diagnostics) manufacturers. Europe follows closely, supported by an active biotechnology sector and widespread adoption of precision diagnostics in national healthcare systems.However, the Asia-Pacific region is witnessing the fastest growth, led by countries such as China, India, and Japan. Rapid expansion of diagnostic laboratories, increased investment in biotech research, and the growing prevalence of chronic and infectious diseases are all contributing to rising demand for accurate immunoassay tools. Point-of-care testing (POCT) and decentralized diagnostic models in rural and underserved areas are further pushing the adoption of interference-blocking technologies to ensure result accuracy in variable field conditions. Additionally, pharmaceutical companies involved in biologics and biosimilar development rely heavily on precise immunoassays, driving demand for interference blockers in drug discovery and clinical trials.
The Growth in the Immunoassay Interference Blocker Market Is Driven by Several Factors…
The growth in the immunoassay interference blocker market is driven by several key factors tied to increasing assay complexity, the expansion of diagnostic testing, and the rising need for accuracy in high-stakes medical decision-making. A major growth driver is the proliferation of immunoassays in disease screening, chronic disease monitoring, and infectious disease diagnostics - where assay reliability is non-negotiable. As the sensitivity of assays improves, their vulnerability to subtle interferences also increases, necessitating robust blocker systems to maintain accuracy.Additionally, the rise of multiplex immunoassays and miniaturized POC diagnostic platforms has created demand for blockers that perform consistently across multiple analytes and assay formats. The ongoing development of biologic therapies and personalized medicine also relies heavily on precise biomarker quantification, reinforcing the need for effective interference mitigation during both research and routine testing. Regulatory scrutiny - especially by the FDA, EMA, and ISO-certified labs - is pushing manufacturers to demonstrate assay performance in the presence of known interferents, making blockers a core part of assay validation.
Finally, growing awareness among clinical labs and diagnostic developers about the economic and clinical risks of misdiagnosis is leading to wider adoption of interference blockers. This includes both integrated solutions in commercial kits and standalone blocker products for use in customized assay protocols. Combined with R&D advances and the global expansion of quality-focused diagnostic testing, these drivers are ensuring that immunoassay interference blockers remain a critical component of modern diagnostics.
Report Scope
The report analyzes the Immunoassay Interference Blocker market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Antibody Immunoassay Interference Blocker, Detection Immunoassay Interference Blocker, Surface Immunoassay Interference Blocker); Technology (Enzyme-Linked Immunosorbent Assay Technology, Chemiluminescence Immunoassays Technology, Fluorescence Immunoassays Technology, Latex Agglutination Technology, Other Technologies); Application (Sandwich Immunoassay Application, Competitive ELISA Application, Other Applications); End-Use (Biotechnology Companies End-Use, Contract Research Organizations End-Use, Academic & Research Institutes End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antibody Interference Blocker segment, which is expected to reach US$236.8 Million by 2030 with a CAGR of a 5%. The Detection Interference Blocker segment is also set to grow at 6.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $81.3 Million in 2024, and China, forecasted to grow at an impressive 8.4% CAGR to reach $81.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Immunoassay Interference Blocker Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Immunoassay Interference Blocker Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Immunoassay Interference Blocker Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, ADx NeuroSciences NV, Banyan Biomarkers, Inc., bioMérieux SA, Bio-Rad Laboratories, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Immunoassay Interference Blocker market report include:
- Abcam plc
- AMS Bio LLC
- AMS Biotechnology (Europe) Limited
- Aviva Systems Biology Corporation
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Candor Bioscience GmbH
- Danaher Corporation
- Electron Microscopy Sciences
- GenScript Biotech Corporation
- Jackson ImmunoResearch Inc.
- Meridian Bioscience, Inc.
- Nordic Biosite AB
- PerkinElmer, Inc.
- Roche Diagnostics GmbH
- Rockland Immunochemicals, Inc.
- Santa Cruz Biotechnology, Inc.
- Scantibodies Laboratory, Inc.
- Surmodics IVD, Inc.
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc
- AMS Bio LLC
- AMS Biotechnology (Europe) Limited
- Aviva Systems Biology Corporation
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Candor Bioscience GmbH
- Danaher Corporation
- Electron Microscopy Sciences
- GenScript Biotech Corporation
- Jackson ImmunoResearch Inc.
- Meridian Bioscience, Inc.
- Nordic Biosite AB
- PerkinElmer, Inc.
- Roche Diagnostics GmbH
- Rockland Immunochemicals, Inc.
- Santa Cruz Biotechnology, Inc.
- Scantibodies Laboratory, Inc.
- Surmodics IVD, Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 470 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
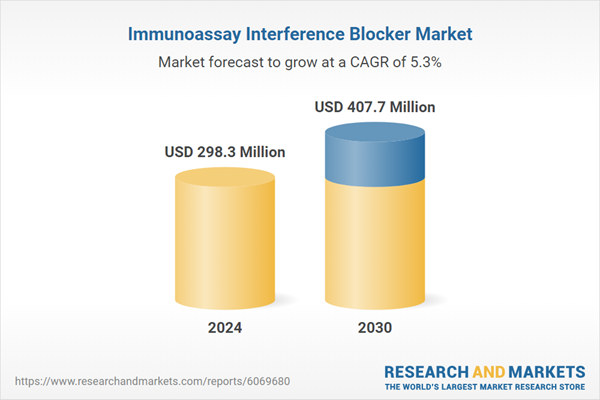
| Estimated Market Value ( USD | $ 298.3 Million |
| Forecasted Market Value ( USD | $ 407.7 Million |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |